Lawn & Garden

Mole crickets have become the most destructive insect pest on turf and lawns in Gulf Coast states. Damage and replacement costs for turf and pastures are annually in the millions of dollars. Learn about the biology, ecology, and management of mole crickets with helpful illustrations and charts.
Pest Mole Crickets and Their Cousins
The insect order Orthoptera includes crickets, grasshoppers, and mole crickets. Within this order, grasshoppers are a separate subgroup from the field crickets and mole crickets. Crickets (such as the field cricket Gryllus spp.) are related to mole crickets but do not live in soil.
Two families of crickets have the common name of mole crickets. Pest mole crickets have digging front legs and live most of their lives in soil, similar to the mammalian mole. Pygmy mole crickets, much smaller and unrelated to pest mole crickets, are not associated with damage to turf or pasture grasses.
What people commonly know as mole crickets (family Gryllotalpidae) in North America are represented by ten species in three genera: Neocurtilla, Gryllotalpa, and Neoscapteriscus (formerly listed as Scapteriscus). Of these, Neoscapteriscus spp. is the common pest species in the southeastern United States. Gryllotalpa major and Neocurtilla hexadactyla are native and, on rare occasions, associated with damage to grass. Interestingly, in the early 2000s a campaign began in England to protect European native mole crickets (Gryllotalpa gryllotalpa) from extinction. Loss of grassland habitat is partially blamed for losses of this native species.
Brief History of Mole Crickets in the United States
Scapteriscus mole crickets were not known to occur in North America before the early 1900s. Three species in the genus Neoscapteriscus were introduced near the Georgia and Florida border from South America.
The short-winged mole cricket (N. abbreviatus) is the least known of these species. It is incapable of flight due to its shortened wings, and it basically has established only in Florida. Two additional species, the tawny mole cricket (Neoscapteriscus vicinus) and the southern mole cricket (Neoscapteriscus borellii, formerly N. acletus) spread and continue to spread across the Gulf Coast states and north along the Carolina coast. Neoscapteriscus also are reported from isolated locations in western states. In the southeastern United States, mole crickets are most likely tawny and southern mole crickets.
Description of Neocapteriscus Mole Crickets
Adult mole crickets are large (1 to 1 1/4”) with elongated bodies. The front pair of legs bear dactyls and the hearing organ called the tympanum, which is analogous to human ears (Figure 1). Neoscapteriscus mole crickets have two clawed dactyls on their forelegs that separate them from the native species, which have four claws. They have long antenna and strong, digging forelegs. Nymphs resemble adults but are smaller in size and lack fully grown wings.
At maturation, the front wings of southern mole and tawny mole crickets are folded back and almost reach the tip of the abdomen. Coloration of the pronotum and the dactyls on the forelegs can be used to differentiate between the other Neoscapteriscus species.
Tawny mole crickets are typically golden brown with a mottled coloration on the pronotum (Figure 2). Southern mole crickets are grayish with four pale dots on the pronotum (Figure 3).
Southern mole crickets are the only species that have four pale dots, although individual southern mole crickets may lack these dots.
Since pronotum color can vary, the appearance of the tibial dactyls is a more reliable characteristic to separate these two species. The tawny mole cricket has a V-shaped space between the dactyls; that is, the dactyls are close together at the base, generally narrower than the width of one dactyl. The southern mole cricket has a U-shaped space (Figure 1); that is, the base of the dactyls is more widely spread (about the width of one dactyl).
Click on images below for full scale
- Figure 1. The hearing organ on the mole cricket is analogous to human ears.
- Figure 2. Tawny mole crickets are typically golden brown with a mottled coloration on the pronotum.
- Figure 3. The southern mole crickets are grayish with four pale dots on the pronotum.
Life Cycle and Damage
All Neoscapteriscus mole crickets produce one generation per year. The exception is in South Florida, where two or more generations may occur, especially for short-winged and southern mole crickets.
Mole crickets spend their lives in soil, with occasional mating and dispersal flights as adults. Mating and dispersal flights take place February to March for tawny and southern mole crickets. Flight occurs within 1 to 2 hours following sunset. Males sing (call) to females from a special chamber in the ground. Females respond to the calls that are unique to the males of their species. (This is the basis for an acoustic trap used mainly for mass collection of adults for research.)
Fall flights for dispersal are also common. Flights and egg laying may continue for sixty or more days, with peak egg laying for tawny and southern mole crickets occurring when soil temperatures are around 75 degrees F. Tawny mole crickets will complete most flight activity before egg laying, but southern mole crickets may fly between egg-laying events.
Soil moisture is a good predictor of egg-laying success and may trigger egg-laying behaviors. Eggs are laid in clutches of ten to sixty eggs (average is thirty-five) in a chamber 1 to 12 inches below the surface, depending on soil moisture (Figure 4).
Egg incubation periods range from 11 to 32 days for tawny mole crickets and from 16 to 37 days for southern mole crickets. Egg incubation is shorter at warmer soil temperatures (≥84 degrees F) and longest when soils are cooler (≤63 degrees F). Longer flight periods and variations in egg incubation times may produce significant variations in population age on a given site (Table 1). Flowering of the herbaceous perennial Agapanthus (Figure 5) is generally a good indicator of peak egg hatch.
Table 1. Average instar collected at each location
Note: Longer flight periods and variations in egg incubation times may produce significant variations in population age on a given site.
| Location | June 15 | August 17 |
|---|---|---|
| #6 | 2nd | 4th |
| #11 | 2nd | 4th range (2-7) |
| #18 | N/A | 9th |
Click on images below for full scale
- Figure 4. Eggs are laid in clutches of ten to sixty eggs in a chamber 1 to 12 inches below the surface, depending on soil moisture.
- Figure 5. Flowering of the herbaceous perennial Agapanthus is an indicator of peak egg hatch.
Mole crickets develop from eggs to adults through a series of seven to ten nymph stages, with no pupal stage (Figure 6). After hatching, young nymphs tunnel extensively as they feed and develop rapidly. Nymphs (Figure 7) resemble adults but lack wings, except for small wing pads; wings develop about two molts before the adult form. As they age, nymphs form more permanent tunnels.
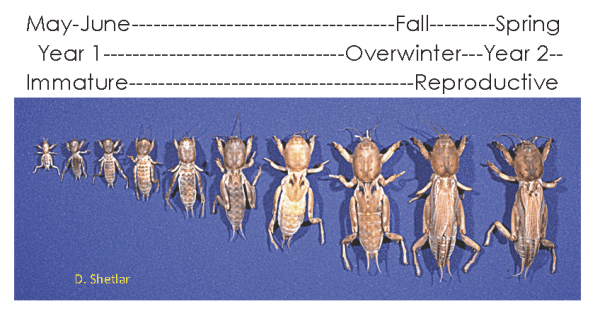
Figure 6. Mole cricket development begins at egg hatch in early summer and is not completed until the insects mature in the spring of the following year. (Image credit: D. Shetlar, The Ohio State University)
Mole crickets are particularly sensitive to desiccation and move in the soil profile in response to changes in soil moisture. There is a significant relationship between soil moisture and damage; even short-term changes in soil moisture due to irrigation can cause damage.
Underground, the tunnels of tawny mole crickets (Figure 8) branch more at the surface with multiple entrance holes. Southern mole cricket tunnels (Figure 9) have one main surface entrance and branch deeper in the soil. More tunneling at the surface likely reflects the more herbivorous nature of tawny mole crickets; whereas, the fewer branching tunnels of southern mole crickets reflects their more carnivorous feeding habit. Both species, however, are omnivores and capable of feeding on both plants and animals (mainly insects). Individuals develop faster when they eat animal tissue as compared to just plants (Figure 10).
Click on images below for full scale
- Figure 7. Nymphs resemble adults but lack wings, except for small wing pads.
- Figure 8. The tunnels of tawny mole crickets branch more at the surface with multiple entrance holes.
- Figure 9. Southern mole cricket tunnels have one main surface entrance and branch deeper in soil.
Tunnels are more extensive in soils that are sandy or loamy sand; they are less complex in areas with mostly clay soils (Figure 11). Tunnels make soils more porous, allowing more surface water to enter the soil profile. In areas with heavy clay soils, tunneling is generally evident only in sand-based greens and tees or sand bunkers.
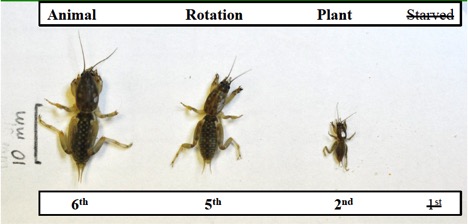
Figure 10. Individuals develop faster when they eat animal tissue as compared to when they eat just plants.
Belowground tunnels erupt to the surface causing numerous small mounds of soil (Figure 12). Tunneling increases as the season progresses. When combined with feeding, extensive tunneling can result in desiccation and susceptibility to other types of damage from foot traffic, golf carts, drought, or possibly pathogens such as Rhizoctonia root rot. Mole crickets feed on grass roots but also feed at night at the surface on grass blades or other insects. Surface- feeding behaviors are the basis for the use of baits discussed later.
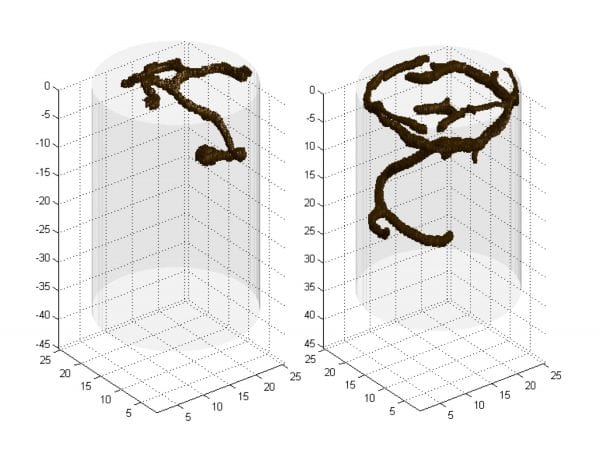
Figure 11. Tunnels are more extensive in soils that are sandy or in loamy sand. They are less complex in areas with mostly clay soils.
Mole crickets spend most of the year as developing nymphs in the soil. Since egg hatching occurs midyear, mole cricket development spans 2 calendar years (Figure 6). By December, mole crickets have developed into adults, and the rest are large nymphs that will complete development in spring of the following year. About 75 percent of southern mole crickets and 15 percent of tawny mole crickets overwinter as nymphs.
On a particular site, mole cricket populations are often a mixture of southern and tawny mole crickets, usually dominated by one species. Fall tunneling activity (September to October) as well as activity in the spring (February to May) may warrant treatment, especially on golf courses. The window for treatment is egg hatch, which varies from May to June depending on location and will be discussed in detail in the management section.
Sampling
Effective management of mole crickets involves sampling for the first occurrence of young nymphs followed by timely use of insecticides. Soap flushing, acoustic traps, and linear pitfall traps serve to directly sample the number of insects per area.
Damage ratings, common in insecticide efficacy tests, serve to measure the amount of damage caused by mole crickets. Testing is performed on 1 x 1 meter squares of net (Figure 13). Multiple evaluations in an area are required to be representative. Damage ratings, developed at Auburn University, are then assigned on a 0 to 9 scale.
Soapy water flushes, also used for sampling caterpillars in turfgrass, and linear pitfall trapping can be used to confirm the presence of nymphs or adults. The soap flush technique is done by mixing 2 tablespoons of liquid dishwashing soap in 1 gallon of water. You then pour the soapy water onto 1 to 2 square feet of infested area (Figure 14). Any mole crickets present will surface in a few minutes. Irrigating the area after flushing can minimize sun scalding of the turf.
Click on images below for full scale
- Figure 12. Mole crickets produce numerous small mounds of soil. They are smaller than fire ant mounds and can be flattened during mowing, which kills the grass underneath.
- Figure 13. Testing for damage ratings is performed on 1 x 1 meter squares of soil.
- Figure 14. Soapy water flushes can be used to confirm the presence of nymphs and adults.
There is no significant difference in the type of soap applied, but lemon-scented detergent is often used. Diluted pyrethrins, a type of insecticide, is actually more effective than soap for causing mole crickets to surface.
Soap flush is 90 percent efficient when soil moisture is ≥19 1⁄2 percent; efficiency drops significantly with decreasing soil moisture. Practically, 20 percent soil moisture means loose sand or sandy loam soils will form a ball when squeezed in your hand, leaving finger marks. If the soil doesn’t hold together, it is likely too dry for an efficient soap flush. For this reason, soap flushes are more successful in irrigated grass or applied in the morning when soil moisture is higher.
Linear pitfall traps are cumbersome to install and have limited use in closely mowed turf applications. Larger nymphs are more readily detected in these traps versus younger nymphs. Low densities (two or three nymphs per 10 square feet) are undetectable with linear pitfall traps. In Florida, nymph captures in linear pitfall traps peak between June and August. Captures of ten to twelve mole crickets per week per trap indicates a significant infestation.
Acoustic traps also are cumbersome tools and, therefore, are used primarily by researchers to collect large numbers of mole crickets for research or to monitor population dynamics. Males and females are attracted to these traps. More males typically land outside of the trap rather than inside it, so the acoustic trap is not very efficient. Acoustic traps also recruit insects from more than a mile away under favorable conditions. Mass recruitment, general inefficiency, and the inability to detect immatures at egg hatch make an acoustic trap virtually useless for pest management.
Management
Biological control efforts were led regionally by the University of Florida. Researchers selected and successfully released a fly, a wasp, and an entomopathogenic nematode. The collective activities of these natural enemies over a 20-year period have been attributed with the reduction in mole cricket captures in Florida traps. Florida is the only state, however, where all these natural enemies are documented to co-exist.
The entomopathogenic nematode Steinernema scapterisci was available commercially as Nematac S (Becker Underwood) but now appears to no longer be in production. Other nematodes such as Steinernema scapterisci and Heterorhabiditis bacteriophora are commercially available for turfgrass pests, but they have limited activity against mole crickets.
Nematodes that are plant parasitic on grass roots are different from those that attack insects. Insect- parasitic nematodes do not infect plants. Mole cricket infection must occur for these nematodes to survive. Nematodes in the infective juvenile stage enter the insect and introduce a specific bacterium that enables the nematode to reproduce and develop. Once the mole cricket dies, new nematodes then exit the cadaver to find new mole crickets to infect. Entomopathogenic nematodes that attack adult mole crickets can be applied in the spring before females lay eggs.
Nematode products are exempt from pesticide registration, but the label specifies how to use the product for maximum infectivity. Typically, they are applied to infested turfgrass in the early morning or late in the day to avoid the heat, which may cause mortality. Many products also may require infested sites to have either moist soil or be irrigated pretreatment followed by post-treatment irrigation.
Nematodes are applied at billions of infective juveniles per acre, making this approach generally more expensive than conventional insecticides. Successful infection of mole crickets will likely not reduce all damage from them, but S. scapterisci may persist at low levels from year to year after the initial treatment. For this reason, entomopathogenic nematodes provide a biological option for homeowners that only want organic or nonchemical controls.
Survival and infectivity of S. scapterisci are generally unaffected when mixed with certain insecticides. Therefore, nematodes, when available, can be used in an integrated pest management program for mole crickets.
The fungal pathogens Beauveria bassiana, Metarrhizium anisopliae, Sorosporella uvella, and Entomophthora sp. are reported from field populations of mole crickets. Of these, strains of Beauveria bassiana have been evaluated as a microbial insecticide in the laboratory and under field conditions with limited success. One limitation appears to be identification of strains of these bacteria that are more specific to infecting mole crickets.
The Larra bicolor wasp (Figure 15) has spread across Florida and is now established in Alabama, Georgia, and Mississippi. Although mainly reported from golf courses along the coast, this wasp can be found as far north as Lee County, Alabama, and on the eastern Atlantic Coast near Myrtle Beach, South Carolina, and Scotland County, North Carolina.
The day-active wasp enters the mole cricket tunnel and forces the cricket to the surface during the day, a behavior that is unusual for mole crickets. At the surface, the wasp stings the cricket, inducing a paralysis that lasts just a few minutes. The wasp then proceeds to lay an egg on the underside of the cricket (Figure 16). The egg hatches and the larva develops externally on the insect (Figure 17), completely consuming the insect before it forms a pupa (Figure 18).
Click on images below for full scale
- Figure 15. Larra bicolor wasps hunt mole crickets in the soil. When not hunting, they feed on nectar of flowers.
- Figure 16. A sting from Larra bicolor will paralyze the mole cricket to lay an egg (shown here) on the underside of the insect.
- Figure 17. The egg hatches and the larva develops externally on the insect.
In the northern Gulf region, the wasp is active from June to November. It uses certain wildflowers and ornamental plants, such as white-flowered Pentas lanceolata, as nectar sources. Access to nectar sources increases the longevity of these wasps and presumably the impact on local mole cricket populations, although this has not been evaluated in the field.
Females can lay two or three eggs per day over their 3-week life span. Since two or three generations of
the wasp occur per year versus a single mole cricket generation, mole cricket mortality is likely to compound locally. Success of this wasp in locating and attacking mole crickets is still dependent, however, on the number of mole crickets in an area and the proximity of those areas to preferred flowering plants, such as pentas or Spermacoce verticillata, where it can obtain nectar. Flowering pentas is an available annual plant, while Spermacoce is naturalized on roadsides and other areas in Florida but not currently available in the nursery trade.
Other natural enemies of mole crickets include southern mole crickets, ground beetles, and vertebrates such as armadillos and birds. In the lab, southern mole crickets will readily consume southern and tawny mole crickets. Lab data supports field observations that indicate southern mole crickets are typically prominant when both species are present.
Mole crickets that eat other insects develop much faster than those that consume just plant parts. Imported fire ants and other ants are voracious predators in turfgrass and are noted to attack mole crickets when active above ground (Figure 19). But predation by imported fire ants on mole crickets in the soil is not well documented.
Predatory ground beetles can also be important predators of mole crickets. Larvae of the ground beetle Pheropsophus aequinoctialis appear to be specialist predators of mole cricket eggs, and female beetles prefer to lay eggs in areas with active tunnels.
A predatory assassin bug, Sirthenea carinata, attacks field crickets, but older nymphs and adults prefer to feed on mole crickets, if available. These large, colorful insects are conspicuous, particularly at night near lights or in the early morning.
In lawns or fairways with a history of mole cricket infestations, insecticide treatment may be preventive to avoid significant damage from animal digging. Armadillos as well as birds may forage in turf for mole crickets. Animals often produce greater damage to grass than mole crickets (Figure 20).
Click on images below for full scale
- Figure 18. The larva completely consumes the insect before it forms a pupa.
- Figure 19. Imported fire ants and other ants attack mole crickets when active aboveground.
- Figure 20. Animals foraging often produce greater damage to grass than mole crickets.
Grass resistance research has failed to identify a grass or grasses that are resistant to mole crickets. Hybrid bermudagrasses, common bermudagrass, bahiagrass, zoysiagrasses, and centipedegrass are most severely damaged. Most of this work has been conducted coincident with breeding programs using captive insects and sometimes natural populations in the field. These studies provide relative rankings, from tolerant to susceptible, for most warm season grasses. For example, TifSport hybrid bermudagrass along with Emerald, Cavalier, and Palisades zoysiagrasses are considered tolerant to tawny mole crickets, while Meyer zoysiagrass and virtually all cultivars of bahiagrass are very susceptible.
Because of the limited number of available biological controls and little success with host plant resistance, chemical control is the primary option for mole cricket management. Mole cricket control depends on the season of the year and the life stages that the pests are in at the time.
Although marketing campaigns make promises and guarantees, mole cricket control is commonly not a one- time application. Timing of controls, life stages present, pesticide formulation, and active ingredient are important considerations. Timing and duration of the control following application is important for the success of the materials used and to prevent selection for resistance.
Table 2. Expected Length of Control by Month of Application for Products Evaluated in Replicated Experiments.
bA blank space indicates no publishing testing in that timing. A “0” indicates that the insecticide was tested during that month but failed to provide control under the experimental conditions.
Be sure to follow label directions precisely and apply only to registered sites as directed.
The months mentioned in this table are the month of application followed by the number of experiments in parentheses.
| Active Ingredient | Example Producta | April (1) | May (2) | June (11) | July (13) | August (6) | Sept. (4) | Oct. (2) |
|---|---|---|---|---|---|---|---|---|
| acephate | Orthene | 2 weeks | 6 weeks | 5 weeks | 2 to 4 weeks | 4 weeks | ||
| bifenthrin | Talstar | 2 weeks | 10 weeks | 6–8 weeks | 6–12 weeks | 3 weeks | 0 | |
| chlorpyrifos | Dursban | 7 weeks | 4 weeks | |||||
| clothianidin | Arena | 6–17 weeks | ||||||
| clothianidin + bifenthrin | Aloft | 10 weeks | 12–20 weeks | |||||
| deltamethrin | DeltaGard | 2–9 weeks | ||||||
| fipronil | TopChoice | 10 weeks | 15–20 weeks | 6 weeks | ||||
| imidacloprid | Merit | 8 weeks | 6 weeks | 4 weeks | 2 weeks | |||
| imidacloprid + bifenthrin | 6-15 weeks | |||||||
| idoxacarb | Provaunt | 4 weeks | 2 weeks | |||||
| lambda-cyhalothrin | Scimitar | 10 weeks | 4 weeks | 0 | ||||
| thiamethoxam | Meridian | 8–17 weeks | 5 weeks | |||||
| trichlorfon | Dylox | 0 |
Depending on when a product is applied, the expected length of residual control may vary. Table 2 summarizes about forty experiments with surface-applied insecticides (excluding baits) by the month of the application. This estimate is based on the average number of weeks that the treatment was significantly lower than the untreated control plots. These experiments were conducted across a range of conditions, formulations, manufacturers, states, and differences in mole cricket ages.
Fipronil, the industry standard for professional turf managers, has the longest residual (15 to 20 weeks) when evaluated in the standard timing. Following application, fipronil is metabolized in the soil, and the metabolites appear to be biologically active (repelling and killing tawny mole cricket nymphs).
In a controlled greenhouse experiment, fipronil and these metabolites were active in soil against tawny mole cricket nymphs for 120 days after application. Repellency of mole crickets by insecticides had been suspected from field observations with limited research support. In the field, mole crickets often tunnel in the untreated borders between treated plots in chemical control studies; this suggests repellency.
Products with active ingredients of clothianidin, clothianidin with bifenthrin, and imidacloprid with bifenthrin reportedly provide a similar residual control as fipronil. This has been further verified in lab tests. In the lab, toxicity of imidacloprid, bifenthrin, and imidacloprid with bifenthrin mixtures actually act faster on the nervous system than fipronil.
The perceived effectiveness of insecticides is often determined by reduced surface activity, which is attributed to mortality as well as avoidance of treated areas. Insecticides that require a contact exposure and those that cause higher mortality are more likely to create avoidance behavior. Insecticides that must be ingested (chlorantraniliprole and perhaps other systemic insecticides) are less likely to reduce surface tunneling due to avoidance.
Synergism (increased effectiveness when multiple insecticides are used in combination) has been documented when neonicotinoid (4A) and pyrethroid (3A) types of insecticides are applied together. Products that exploit this insecticide synergy are currently available for homeowners (Bayer Complete product line) and professionals (e.g., Triple Crown). Despite being different chemicals, clothianidin and imidacloprid have a common mode of action and may be problematic if used on the same site.
The Insecticide Resistance Action Committee (www.irac-online.org) classifies all insecticide modes of actions and chemical classes according to a number and letter (combination 22A, for example). To manage resistance, you want to use products that have a different number (Table 3). Insecticide resistance has not been documented in mole crickets, but multiple applications of insecticides against a single generation of insects can select for resistance.
Table 3. IRAC Chemical Codes
| Active Ingredient | IRAC Code |
|---|---|
| acephate | 1B |
| bifenthrin | 3A |
| carbaryl | 1A |
| chlorpyrifos | 1B |
| clothianidin | 4A |
| cyfluthrin | 3A |
| dealtamethrin | 3A |
| dinotefuran | 4A |
| fipronil | 2B |
| imidacloprid | 4A |
| indoxacarb | 22A |
| lambda-cyhalothrin | 3A |
| permethrin | 3A |
| thiamethoxam | 4A |
| trichlorfon | 1B |
| zeta-cypermethrin | 3A |
There are typically three times in a 12-month period (early spring, egg hatch, and fall) when mole cricket damage may warrant treatment (Figure 21). Overwintered mole crickets become active in February to April. Treatment at this time is optional, except in highly maintained turf areas or sod fields. Early spring treatment reduces tunneling damage at that time but usually does not replace treatment later in the season. This timing is important because individuals that may survive this treatment will then mate and produce eggs within 3 to 4 months.
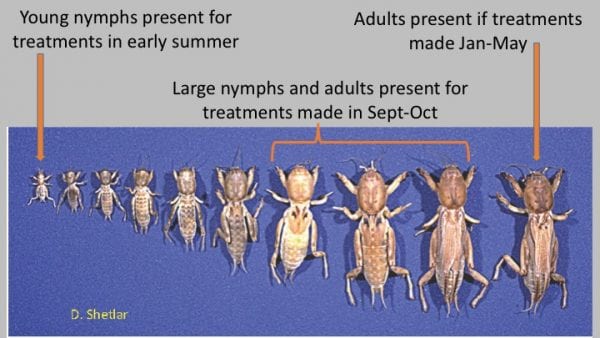
Figure 21. “Mole cricket damage may warrant treatment at egg hatch and in fall of one year and again in spring (about February) of the following year. (Image credit: D. Shetlar, The Ohio State University)
Internally, mole crickets have enzyme activities that enable them to detoxify insecticides. Related to timing, spring populations tend to express more of these enzymes than do fall populations. This further illustrates the need to be mindful in applying treatment. The action modes of products used in spring are different from those used later.
The primary application timing for mole cricket control with insecticides is when young nymphs hatch from eggs. This happens between early May and mid-June, depending on weather and location. Full bloom of Agapanthus lilies should prompt you to begin soap flushes. Treatments on the more vulnerable nymphs in June, July, and even early August are more effective than later treatments on larger mole crickets.
It is sometimes difficult to convince homeowners to treat in late June or July because there is little (if any) evidence of the spring’s mole cricket damage by that time. If mole crickets are active in an area during March, April, and May, there are usually treatable populations of new-generation nymphs that hatch there in June and July.
By the time mole cricket damage is visible, control efforts are more difficult. This is further complicated by use restrictions for products used on urban lawns or products only available for professional use (fipronil, for example). In most cases, the number of available products for use by homeowners (Table 4) or labeled for use on lawns are fewer than those available to professionals. See Integrated Pest Management Guides for the Extension publication IPM-1313, “Commercial Turf and Lawns, Insect Control Recommendations.”
Table 4. Products Available for Mole Cricket Control by Homeowners
| Active Ingredient | Examples (trade or brand names) | Comments |
|---|---|---|
| bifenthrin | Talstar | Granular and spray forms require irrigation after application. |
| carbaryl | Carbaryl bait | Do not irrigate after application. |
| deltamethrin | Turf Ranger Insect Control granules | Granular form that requires irrigation after application. |
| gamma-cyhalothrin | Triazicide Insect Kill concentrate | Granular and spray forms require irrigation after application. |
| imidacloprid + cyfluthrin | Bayer complete insect killer | Granules and spray forms. |
| lambda-cyhalothrin | Demand G | Granules should be irrigated after application. |
| permethrin | Various | Requires irrigation after application. |