Fish & Water

No two ponds are ever exactly alike. Even ponds in the same watershed and built very close to each other can respond very differently to management approaches. Differences in soil, surrounding vegetation, the amount of water runoff, and the depth of the pond can dramatically influence how the pond responds to management practices.
Water Quality
Water-quality factors of temperature, pH, alkalinity, hardness, and dissolved oxygen affect fish health and production. These factors are constantly changing in a pond. Temperature, dissolved oxygen, and pH differ as seasons change and will even change or cycle each day.
Oxygen Cycle
Oxygen is dissolved in water from two sources—air and photosynthesis. Oxygen dissolves into the pond water from the air as the two are mixed together through wind and wave action. Mechanical aeration using pumps, sprayers, and paddle wheels can be used to increase dissolved oxygen levels during periods of low oxygen.
Photosynthesis is the largest source of dissolved oxygen in ponds. In this process, plants and algae produce oxygen while making food from carbon dioxide and water in the presence of sunlight.
Algae release oxygen directly into the water during photosynthesis. Since photosynthesis is driven by the energy of sunlight, oxygen production occurs during daylight. Dissolved oxygen concentrations in ponds, therefore, tend to rise throughout the day. At night dissolved oxygen slowly declines as fish, insects, zooplankton, bacteria, plants, and algae consume oxygen through respiration.
Under normal conditions, dissolved oxygen concentrations should not fall below 3 to 4 parts per million (ppm). Oxygen concentrations below 3 ppm stress fish, and many fish will suffocate at concentrations below 2 ppm. Ponds typically reach minimum dissolved oxygen just before dawn.
Alkalinity, Hardness, and pH
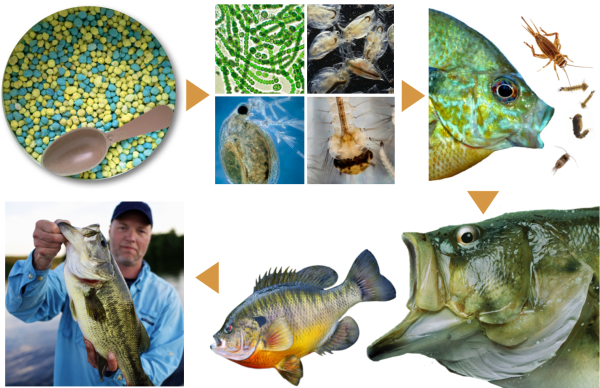
Pond Food Chain
Alkalinity and hardness are important in providing adequate natural food and maintaining a healthy fish population. The pH of the pond cycles daily because of respiration and photosynthesis.
Carbon dioxide released from respiration reacts with water, producing carbonic acid. The pH scale measures the acidity; therefore, as carbonic acid is formed, the pH is lowered, meaning the pond becomes temporarily more acidic.
Algae use carbon dioxide for photosynthesis during daylight hours, and the pond water becomes less acidic with the decline of carbonic acid. Because of this, pond pH normally fluctuates between 6.5 and 9. If the pH drops below 5, as it does in ponds that receive acidic runoff, or rises above 10, as in low alkalinity ponds with excessive algae blooms, fish will be stressed and can die. The only practical method to use for abnormal pH changes is to increase the alkalinity of the pond.
Alkalinity is a measure of bases in the water. Bases react to neutralize acids and, therefore, directly influence pH. As bases react with the hydrogen ions present, they buffer or suppress pH changes. Alkalinity of 20 ppm or more is necessary for proper algae growth and good fish production.
Hardness is a measure of calcium and magnesium ions. Hardness concentrations are usually similar to alkalinity, if derived from limestone, but can be different in coastal areas especially. A lack of hardness can reduce plankton production and increase the likelihood of muddy pond water.
Blooms and Pond Color
Plankton is a term used for all microscopic and near- microscopic living things that float in the water. Plankton includes both tiny algae or phytoplankton and animals called zooplankton.
Planktonic algae are the most important part of the base of the food chain. Zooplankton and aquatic insects feed on algae. They in turn are eaten by small fish (fry), which are then eaten by larger fish. Algae directly or indirectly provide almost all the basic food for the pond except for a small number of insects and worms that fall or wash into the pond. Managing planktonic algae is essential in providing food to produce an abundant and healthy fish population.
Pond water color and clarity are strongly affected by the amount of planktonic algae, called “blooms,” or to suspended sediments and organic matter. Water that is good for fish production is green. The green color comes from billions of suspended microscopic algae. Water color changes if these algae blooms die off rapidly, turning the water brown, black, milky, or clear. When this happens decomposition of the dead algae consumes oxygen that can cause fish stress or death.
There are many factors that tend to cause algal bloom die-offs. Among these are ponds with low alkalinity and hardness. Algae die-offs are common in deep hill-type ponds or in fish ponds receiving too many nutrients. Mechanical aeration may be necessary after algae die- offs to keep fish alive.
Sediments washed into ponds after heavy rains will also change pond color. Color should return to normal within a few days as settling occurs. Heavy sediment loads can stress fish and other animals, shade out and prevent algal blooms, bind fertilizer, and make certain herbicides ineffective.
Ponds that receive sediments from surrounding fields need a protective vegetation strip around them to help trap the sediments before they enter the pond. A pond that receives sediment only during heavy rains may need a diversion ditch built around it to channel excess water away from the pond. Many chronically muddy ponds need lime to reduce acidity, increase hardness, and settle suspended clay. If your pond is always muddy, contact your county Extension agent for help.
Read more excerpts from Management of Recreational Fish Ponds at www.aces.edu.
 Rusty Wright, Extension Specialist, Associate Professor, Fisheries, Aquaculture, and Aquatic Services, Auburn University
Rusty Wright, Extension Specialist, Associate Professor, Fisheries, Aquaculture, and Aquatic Services, Auburn University
Revised May 2022, Management of Recreational Fish Ponds, ANR-0577