Lawn & Garden

*This is an excerpt from The Alabama Vegetable Gardener, ANR-0479.
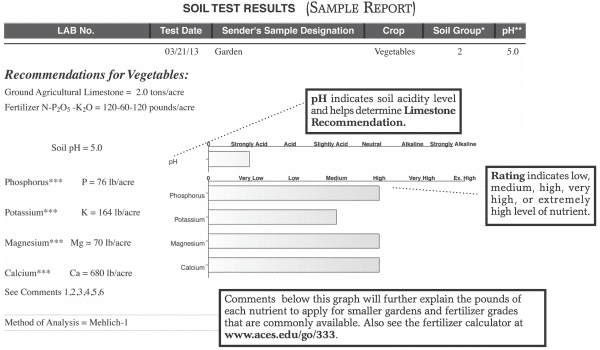
Acid soils (low pH) cause more gardening problems in Alabama than any other soil factor.
Soils become acid naturally in humid climates. Rainfall, over time, leaches out the basic minerals in soils. Fertilizers and organic matter from manure and compost tend to speed up this process. Under very acid conditions (below pH 5.3), some minerals such as aluminum and manganese become very soluble and can be toxic to plants. Plant nutrients such as calcium and magnesium may be deficient in acid soils. Beneficial soil bacteria that fix nitrogen on the roots of legumes such as beans and peas cannot survive in acid soils.
Most Alabama garden soils will require liming every 3 to 5 years to maintain the soil pH between 5.5 to 6.5. A detailed soil test by a reputable laboratory is the only way to determine the precise soil pH and lime requirement.
Soil Acidity and Its Effect
| Soil | Soil pH | Effect |
|---|---|---|
| Extremely acid | below 4.5 | Very few crops survive; aluminum/manganese toxicity. |
| Very acid | 4.5–5.0 5.0–5.5 | Only acid-tolerant plants such as azaleas and blueberries do well. Some aluminum and manganese toxicity; nutrient deficiencies. Ideal pH for Irish potatoes because scab bacteria doesn’t survive well at this pH. Most crop yields slightly reduced. |
| Moderately acid | 5.5–6.0 | No visible problems with most crops; yields of crops requiring high calcium and magnesium may be reduced (for example, tomatoes and peppers). |
| Slightly acid | 6.0–7.0 | Ideal for most crops; best for soil bacteria/nitrogen fixation. Optimum nutrient availability. |
| Slightly alkaline | 7.0–8.0 | Micronutrient deficiencies of iron, zinc, and manganese may occur; too high for acid-loving crops. |
| Moderately alkaline | 8.0+ | Severe micronutrient deficiencies. Few garden crops do well. |
For more information, see other excerpts from The Alabama Vegetable Gardener, ANR-0479.
Kerry Smith, Extension Home Horticulture Associate; Ayanava Majumdar, Extension Entomologist; Charles Mitchell, Extension Agronomist, Professor, Agronomy and Soils; John Everest, Visiting Professor, Agronomy and Soils; Edward Sikora, Extension Plant Pathologist, Professor, Entomology and Plant Pathology; Joseph Kemble, Extension Specialist, Professor, Horticulture; all with Auburn University; and Rufina Ward, Research Entomologist, Natural Resources and Environmental Sciences, Alabama A&M University.
Reviewed October 2021, The Alabama Vegetable Gardener, ANR-0479

