Crop Production

Scouting for insect pests is a critical component of an integrated pest management (IPM) system. Scouting involves correct identification of pest species, determination of infestation levels, and reliable estimation of crop damage.
Once scouting confirms the presence of insect pests, a well-planned monitoring program can assist in making management decisions. Remember that “no treatment” is also considered an IPM tactic when treatment is not justifiable.
This document provides information about scouting techniques for some soil-dwelling insect pests of peanuts. It is not, however, an all-inclusive list of insect pests, and sampling methodologies may require modifications depending on location, crop type, growth stage, sampling time, cost, etc. Note that soil sampling is not a perfected method, and a scout may have to check the foliage in conjunction with soil or roots to complement soil sampling. The experience of a scout can also affect the accuracy of sampling.
Various sampling methods including the germinating seed bait technique as well as critical details about overwintering stages and insect behavior are included to assist field scouts. For soil insect pest identification, see “Scouting Techniques for Soil Insect Pests of Peanuts” (ANR-1351) on the Alabama Extension website.
The Alabama IPM pheromone trapping project is a new team endeavor to monitor about a dozen critical insect pests of peanuts (and vegetables) to generate insect advisories for farmers.
Burrower Bugs (Hemiptera: Cydinidae)
About six species of burrower bugs have been reported from peanut farmers in several states. The predominant species is Pangaeus bilineatus, and it may cause direct kernel injury. In the overwintering stage, adult bugs hide under rocks, crop stubble, volunteer plants, and decaying wood for winter protection.
Behavioral Clues
Burrower bugs are opportunistic insects that readily migrate (starting in late June) in search of suitable hosts.
Adult bugs disperse by walking on soil surfaces and may occasionally take flight. Nymphs prefer to follow cracks in soil rather than walking on soil surfaces. This behavior minimizes risk to the insect from predators. Surface and subsurface activities of adults and nymphs are affected by soil moisture levels. Adults may dig deep in the soil to avoid saturated conditions. Adult bugs are attracted to white light. Their high numbers in backyard light could indicate their activity.
Scouting Techniques
- Pitfall traps. These can be set up in the ground relatively inexpensively from May to September to monitor nymphs and adults. Place a medium-sized plastic cup in the soil up to the top rim and fill one- third with water or antifreeze to drown the insects. You can also place wooden garden stakes in the ground in four primary directions; insects usually follow the edge of these stakes and drop into the cup. Check weekly for insects and more frequently during the rainy season. Monitor future peanut fields using such pitfall traps before planting the crop.
- Spade sampling. This could also be useful, but it is a laborious process. Soil samples can be collected with a soil corer (4 to 6 inches diameter, 4 inches deep) and sifted with a mesh screen (size 5 to 12). Sampling is critical during the period of intense burrower bug activity late in the season (i.e., July to August).
- Direct examination of pods. This is the most reliable method of sampling. Start scouting at the R6 (full seed) growth stage. Pods should be partially dried to enhance detection of feeding injury on the kernels.
Whitefringed Beetles (Coleoptera: Curculionidae)
This insect (Naupactus sp.) is a native of South America. It was first reported from Florida in 1936, but it is now present in more than a dozen states; its dispersion has been facilitated by commercial activities. Overwintering stage: Larvae or grubs move to a depth of 10 to 12 inches for overwintering. Grubs move within 3 to 4 inches of soil to pupate. Eggs laid in soil can also overwinter.
Behavioral Clues
Adult females are flightless, so dispersion of this insect between fields is relatively slow. Adults walk between fields and choose optimum sites for oviposition. Beetles feed on leaves and cause characteristic “notching” along the leaf margin. Because all beetles are females, control of adults could substantially reduce egg numbers.
Scouting Techniques
- Soil cores. These should be collected before the peanut planting season and examined for larval populations. However, freshly hatched larvae are difficult to detect in soil because of their small size. Mature larvae can be retrieved from soil using sieves with a 3- to 6-millimeter opening.
- Early detection of adults. This cost- and time- effective technique is one of the most reliable indicators of infestation for this insect. Shake the plant foliage over soil between the rows and watch for insects that drop off. Whitefringed beetles do not fly, so sampling can be done relatively easily.
Southern Corn Rootworms (Coleoptera: Chrysomelidae)
The southern corn rootworm (SCRW), Diabrotica undecimpunctata howardi, is the immature form of the spotted cucumber beetle. Overwintering stage: Beetles diapause in November, but the diapause is short and they could emerge quickly the following season. Research indicates that most overwintering populations consist of female beetles that oviposit the following year.
Behavioral Clues
Larvae do not make webs, so feeding injury from smaller instars may not be evident on the pods. The adult beetle is highly migratory and a strong flyer.
Scouting Techniques
- Larval infestations. Infestations in the soil should be thoroughly scouted close to the pegging stage. Remember that high soil moisture and poorly drained soils are favorable for the buildup of this insect. Initiate control measures when rootworm larvae are found in 30 percent of sample locations.
- Pheromone traps. These can be used to monitor beetle activity. Pheromone traps for this insect are available commercially as easy assembly kits. According to industry sources, trap catches of over 100 beetles per trap per week is indicative of high insect activity that should be investigated further by direct crop scouting and soil sampling for active larvae to estimate pod damage. Note that larval activity in soil may lag behind adult emergence and mating period.
- Sweep netting. This is a reliable sampling method for SCRW beetles. Collect a sample by making ten sweeps over the crop canopy in ten paces, and repeat this step from five locations per acre (collect representative samples from several random locations). Sweep net sampling should be done mid-morning because afternoon heat could move the insects deep into the crop canopy, thereby reducing sampling accuracy. If feasible, check the plant canopy for the actual number of adult beetles present on entire plants and compare the accuracy of sweep net samples.
- Check for holes. Check the pods for small to mediumsized circular holes at one end of the pod. In some instances, larvae make a series of entry holes all in one area of the pod.
- Risk index. Although SCRW infestations are sporadic, peanut producers may use the “risk index” developed by North Carolina State and Virginia Cooperative Extension to gain more experience about risk factors. SCRW risk index incorporates factors such as cultivar resistance, soil texture, soil moisture, field history, and planting date. Action threshold: Presence of adult or larvae or fresh damage in one-third of sampling sites indicates need for treatment. https://digitalpubs.ext.vt.edu/vcedigitalpubs/3345945251386462/MobilePagedReplica.action?pm=2&folio=1#pg1
Lesser Cornstalk Borer (Lepidoptera: Pyralidae)
The lesser cornstalk borer (LCB), Elasmopalpus lignosellus, is one of the major insect pests of peanuts in some parts of Alabama. Overwintering stage: Larvae overwinter in soil and crop debris.
Behavioral Clues
Research indicates that LCB is better able to survive dry soil conditions in which its natural predators cannot thrive. This anomaly results in a greater incidence of LCB in the soil. Larvae of this insect are highly attracted to carbon dioxide and heat released by belowground plant parts of peanuts.
Scouting Techniques
- Scouting maps. It is very important to detect this insect early in the season so that control measures can be undertaken in a timely manner. Scouting maps are available for LCB through the AWIS Weather Services Web site. Based on calculation of borer days (BD), there will be a need to scout if the model has values within a 0 to 5 range. BD values over 5 indicate unusually high larval populations.
- Pheromone traps. These are highly effective in detection and monitoring of flight activity of adults. Preliminary data from the 2009 IPM pheromone trapping project in Alabama indicate that moth flight initiate in June followed by several weeks of intense mating activity in July and August. High and dry areas in sandy fields should be monitored closely for crop injury because LCB larvae survive well under xeric conditions.
- Look for webbing. Pull some pods out of the ground and look for webbing attached to damaged pods. Larvae make feeding tubes that can be easily detected. Action threshold: Presence of larvae in 30 percent of sampling sites suggests economically injurious populations.
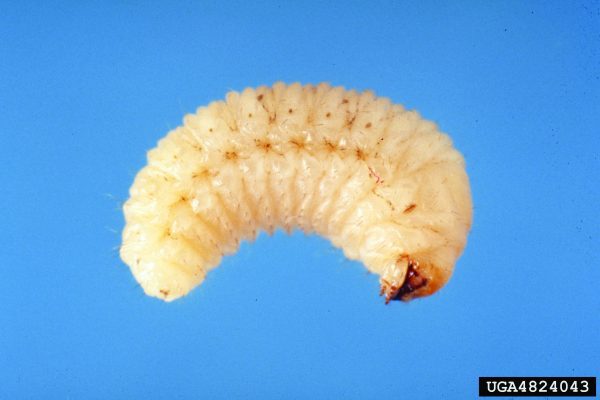
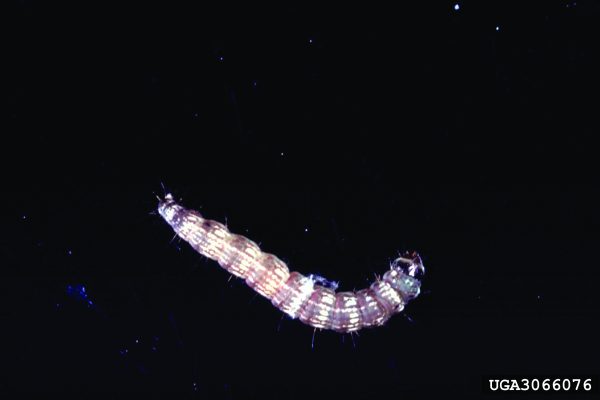
Wireworms (Coleoptera: Elateridae)
Wireworms have become the major insect pest of peanuts under certain cropping conditions. Several species of wireworms occur in peanut fields, so an independent description for all species is not provided in this publication. Wireworms can cause significant late-season loss of peanut pods. Overwintering stage: Larvae overwinter in soil and move deep into it to prevent dehydration and reduce threat from natural enemies. Wireworms have an extended life cycle (2 to 9 years), depending on the species.
Behavioral Clues
Good mobility of larvae makes management of the insect very difficult. Larvae prefer soil moisture in the range of 6 to 16 percent and become immobile at low soil temperatures. Wireworms are also infamous for their behavioral resistance (i.e., many species have the ability to avoid insecticide-treated areas resulting in control failure).
Scouting Techniques
- Sampling. Wireworm larvae make a large entry hole on the distal end of pods. Pod injury could be highly clumped (nonuniform), so draw a large number of pod samples from different parts of the field for high sampling accuracy.
- Germinating seed baits. Fields with a history of infestations should be intensively scouted before planting. The most reliable sampling method is with germinating seed baits placed in soil. Wireworms are attracted to the carbon dioxide and heat released by germinating seeds in ground baits. Note that the attractiveness of seed baits varies with wireworm species (e.g., corn/wheat seed bait are attractive to Agriotes and Melanotus sp., and sorghum seed baits are attractive to Conoderus sp.).
- Pitfall traps. These have been very effective for catching surface-dwelling click beetles (i.e., adult wireworms). Several pitfall traps (plastic cups filled one-third with soapy water) should be deployed in suspected fields, especially between the peanut rows, and traps should be marked with tall flags to increase visibility. Action threshold: One or more wireworms from each bait station indicate a need for treatment.
Germinating Seed Bait Technique for Intensive Sampling Advantages
Advantages
The germinating seed bait technique has been shown to be the most cost-effective relative sampling technique for a variety of subterranean insect pests. The seed bait technique can complement or replace an absolute sampling method such as spade sampling, which could be laborious and time-consuming in an intensive sampling program. The germinating seed bait technique has been used successfully in agronomic crops, vegetable crops, pasture, and fields under the Conservation Reserve Program; this method is relatively easy to set up and remove.
Materials
Untreated corn/wheat/sorghum seeds, water, black polyethylene trash bag, shovel, large resealable plastic bag (optional).
Method
A mixture of seeds can be used for increasing attractiveness of seed baits to different insect species (especially for detecting wireworms).
- Presoak the seed mixture for a 24-hour period before placement to encourage seed germination.
- Dig a hole about 10 inches wide and 6 inches deep, and put an appropriate amount of seed mixture in the hole.
- Cover the seeds with a shallow layer of soil and put a 15 × 15–inch piece of black polyethylene bag on top of the mound. Use soil to seal the edges of the polyethylene bag. The black cover will trap solar radiation and facilitate seed germination. It also will deter animals from disturbing the bait. You can put colored flags to mark the position of your bait stations.
- Although it is difficult to recommend a specific number of baits per acre, about a dozen or more bait stations in a 40-acre field could be sufficient. Remember, the fewer the number of sampling units, the lower the accuracy of sampling and vice versa.
- Maintain seed baits for at least 1 week in the spring of each year or up to 1 week prior to planting crops. If feasible, seed baits should be deployed throughout the production season in fields with a history of wireworm infestation to monitor peak larval activity.
- At the end of sampling period, dig out the germinating seeds and the soil surrounding the seed bait. Sometimes, wireworms collect just under the seed bait, so collecting surrounding soil improves detection. Carefully go through the sample by manually sorting the bait on a plastic tray or by pressure washing over a mesh.
- For intensive sampling program, the entire sample could be stored in a resealable plastic bag, frozen, and checked at a later date (freezing prevents rapid organic matter decomposition inside the bag).
Note: Attractiveness of seed bait to wireworms is affected by their population distribution and the species present in the field. A number of seed baits could be left in the ground for different time periods in order to trap a large variety of below ground and surface-dwelling insects. It should be noted that scouting with special equipment and design improves with experience of the field personnel. If good records are maintained, then these sampling techniques can complement physical plant observations and provide information about seasonal activity of insects over a number of years. Insecticidal treatments should be applied at the most appropriate time toward the most vulnerable stage of insect life cycle.
 Scott H. Graham, Extension Specialist, Assistant Professor, Agronomic Crops, and Ayanava Majumdar, Extension Entomologist, Agronomic Crops and Commercial Horticulture, and State Leader for Program Evaluation, both with Auburn University
Scott H. Graham, Extension Specialist, Assistant Professor, Agronomic Crops, and Ayanava Majumdar, Extension Entomologist, Agronomic Crops and Commercial Horticulture, and State Leader for Program Evaluation, both with Auburn University
Revised May 2022, Scouting Techniques For Soil Insect Pests of Peanuts, ANR-1364