Crop Production

Too much phosphorus (P) in water stimulates the growth of algae, cyanobacteria, and aquatic plants, which can result in harmful algal blooms, oxygen depletion, and water quality degradation. Monitoring phosphorus levels in water bodies is crucial for assessing the potential risk of eutrophication or problems resulting from the water being too full of nutrients.
Phosphorus is present in water in both organic and inorganic forms. These forms may be present either in a particulate phase (associated with living and dead plankton such as algae, cyanobacteria, and suspended sediments) or a dissolved phase. There remains a lack of awareness and knowledge about the different species of phosphorus found in water and how to quantify them operationally. The different types of phosphorus species discussed in this publication can simplify communication, prevent misinterpretation, and help in targeted management of phosphorus to prevent water quality degradation. As a continuation of the Phosphorus Basics series, this publication can be used by soil and water professionals to identify phosphorus species found in runoff water and freshwater systems. This topic will be relevant for monitoring, managing, and preventing nutrient pollution in aquatic ecosystems.

Figure 1. Sediments flowing in a runoff event.
Phosphorus is an essential macronutrient for crop growth and is applied to agricultural lands through inorganic (chemical fertilizer) or organic (animal manures, compost) sources. Soil organic phosphorus forms include inositol phosphates, phospholipids, nucleic acids, and their derivatives. Soil inorganic phosphorus compounds range from highly soluble phosphates to moderately soluble calcium phosphates and stable iron and aluminum phosphates. Soil phosphorus that is readily available to plants (plant available P) represents a small fraction (less than 5 percent) of soil total phosphorus. High soil phosphorus fixation and slow diffusion result in low phosphorus availability to plants. Most phosphorus is strongly bound in soil particles and partially weathered material or occluded in secondary minerals. For example, in acidic soils, phosphorus can be dominantly adsorbed by aluminum or iron oxides and hydroxides. In neutral to calcareous soils, phosphorus can precipitate with calcium or be adsorbed on the surface of calcium carbonate. External application of phosphorus in agricultural lands has improved soil phosphorus fertility and crop production. However, repeated applications of high phosphorus rates for extended periods lead to the accumulation of phosphorus in surface soils, which are susceptible to loss risk during rainfall events via surface runoff (figure 1) and leaching.
Phosphorus is often the limiting nutrient in freshwater systems for algal and cyanobacterial growth, but concentrations ranging from 0.01 to 0.03 ppm are sufficient to drive eutrophication. Phosphorus in runoff water can be present in both inorganic and organic forms, determining its bioavailability. Inorganic phosphorus in runoff water comprises dissolved inorganic phosphorus (commonly known as dissolved reactive phosphorus or soluble reactive phosphorus) and unavailable forms of inorganic phosphorus associated with the particulate phase. Types of inorganic phosphorus include orthophosphate and polyphosphates. Dissolved reactive phosphorus is readily bioavailable and typically quantified in analytical laboratories by passing the water solution through a 0.45-micron filter paper and analyzing the phosphorus concentration in the filtrate. Dissolved reactive phosphorus consists of inorganic orthophosphate and is used as an index of phosphorus immediately available for primary producers. Organic phosphorus is defined as the phosphate molecule associated with a carbon-based molecule. Considerable portions of dissolved organic phosphorus in natural waters can be readily hydrolyzed by microbial populations and hence considered bioavailable. Although studies have documented dissolved reactive phosphorus as the most bioavailable form of phosphorus in aquatic ecosystems, there is growing interest in understanding organic phosphorus transport, transformation, and bioavailability. Understanding different phosphorus species found in runoff water will be beneficial to implementing management practices to reduce eutrophication. This publication provides information about different phosphorus species found in runoff water (figure 1) and how they can be characterized in the laboratory and operationally identified for targeting water quality improvement.
Phosphorus Species
Phosphorus in runoff water can be categorized into the following species based on filtration or digestion of the water sample:
- Total phosphorus represents the total phosphorus in runoff water and is the sum of dissolved and particulate inorganic and organic forms of phosphorus.
- Total inorganic phosphorus represents the sum of dissolved inorganic phosphorus and inorganic phosphorus from the particulate phase. It is commonly known as total reactive phosphorus.
- Total organic phosphorus represents the sum of dissolved organic phosphorus and organic phosphorus bonded on the particulate phase.
- Total dissolved phosphorus represents dissolved inorganic (dissolved reactive phosphorus) and organic phosphorus (dissolved organic phosphorus).
- Total particulate phosphorus represents the total undissolved phosphorus in the particulate phase and is the sum of organic (particulate organic phosphorus) and inorganic (particulate inorganic or particulate reactive phosphorus) forms.
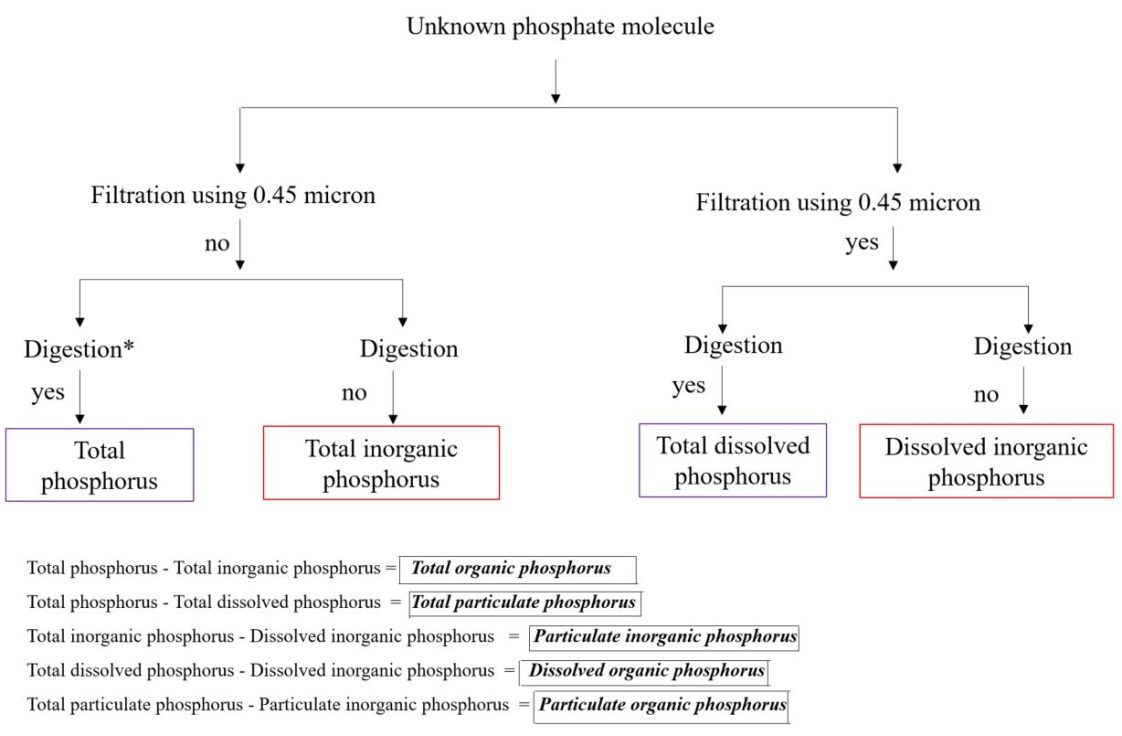
Figure 2. Schematic representation of the different phosphorus species in runoff water based on filtration and digestion method. The digestion of the water samples can be done using an autoclave or microwave-assisted digestion. (Figure credit: Modified from L. Felgentreu, G. Nausch, F. Bitschofsky, M. Nausch, D. Schulz-Bull, and P. Worsfold, I. McKelvie, and P. Monbet.)
The different operationally defined phosphorus species found in runoff water are shown in figure 2.
- Dissolved reactive phosphorus: Represents dissolved inorganic phosphorus.
- Particulate reactive phosphorus: Represents inorganic phosphorus coming from suspended sediments.
- Dissolved organic phosphorus: Represents dissolved organic phosphorus.
- Particulate organic phosphorus: Represents organic phosphorus from suspended sediments.
Out of the nine phosphorus species mentioned above, four species, namely total phosphorus, total inorganic phosphorus, total dissolved phosphorus, and dissolved inorganic phosphorus, are quantified directly using analytical instruments. The remaining five phosphorus species (total organic phosphorus, total particulate phosphorus, particulate inorganic phosphorus, dissolved organic phosphorus, and particulate organic phosphorus) are derived by the subtraction method, as shown in figure 2.
Phosphorus Analysis
Phosphorus species in water can be analyzed using the following methods:
- A spectrophotometer or colorimeter is often and widely used to determine the intensely colored blue phosphomolybdenum complex produced by the reaction of orthophosphate with ammonium molybdate and ascorbic acid 6. The blue color in the sample is directly proportional to the amount of orthophosphate present.
- An atomic emission spectrometer or inductively coupled plasma-optical emission spectrometer (ICP-OES) can also be used to determine the sample’s total phosphorus concentration, especially when the concentration is high.
Conclusion
The operationally defined phosphorus species described in this publication can be used to distinguish different phosphorus species in runoff water. Understanding the different phosphorus species will simplify communication and can be used to implement practical nutrient reduction goals. Eutrophication potential will be misrepresented if all the phosphorus species are not considered effectively. The function of different phosphorus species and their potential role in phosphorus bioavailability is vital for successful management strategies. Monitoring phosphorus species concentrations is a critical step in preserving and restoring the health of freshwater systems.
Reference
1. Dyhrman S. T., Chappell P. D., Haley S. T., Moffett J. W., Orchard E. D., Waterbury J. B. Webb E. A., 2006. Phosphonate utilization by the globally important marine diazotroph Triscodesmium. Nature 439, 68–71.
2. Li, B., Brett, M. T., 2013. The influence of dissolved phosphorus molecular form on recalcitrance and bioavailability. Environ. Pollut. 182, 37–44. doi: 10.1016/j.envpol.2013.06.024
3. S.S. Butcher, R.J. Charlson, G.H. Orians, G.V. Wolfe, Global Biogeochemical Cycles, Academic Press, London, 1992, p. 367.
4. Felgentreu, L., Nausch, G., Bitschofsky, F., Nausch, M., Schulz-Bull, D., 2018. Colorimetric chemical differentiation and detection of phosphorus in eutrophic and high particulate waters: Advantages of a new monitoring approach. Front. Mar. Sci. 5.
5. Worsfold, P., McKelvie, I., Monbet, P., 2016. Determination of phosphorus in natural waters: A historical review. Anal. Chim. Acta 918, 8–20.
6. APHA. 2017. Standard method for the examination of water and wastewater. 23rd ed. American Public Health Association.
 Debolina Chakraborty, Assistant Research Professor, Biosystems Engineering, and Rishi Prasad, Extension Specialist, Associate Professor, Crop, Soil, and Environmental Sciences, Auburn University
Debolina Chakraborty, Assistant Research Professor, Biosystems Engineering, and Rishi Prasad, Extension Specialist, Associate Professor, Crop, Soil, and Environmental Sciences, Auburn University
New March 2024, Phosphorus Basics: Phosphorus Species Disruptive to Freshwater Systems, ANR-3057

