Lawn & Garden

Snap and lima beans are very popular crops for both commercial and backyard production in Alabama. A number of common diseases attack these fresh-market beans from both above and below ground.
Root Diseases
Rhizoctonia Root Rot
Rhizoctonia root rot, caused by Rhizoctonia solani, is common throughout the world. It is one of the most economically important root diseases of beans. It has a broad host range that includes most annual and many perennial plants.
Symptoms
Typical symptoms of Rhizoctonia root rot include small, elongated, sunken, reddish-brown lesions on the roots and hypocotyls of young plants (figure 1). Lesions often occur at the soil line and appear water-soaked initially, then turn dry and reddish-brown to brick-red with age (figure 2).
- Figure 1. Reddish-brown, sunken lesion on bean root caused by Rhizoctonia root rot
- Figure 2. Rhizoctonia root rot lesions near soil-line on beans
Lesions may girdle the seedling and cause stunting and death of plants. Infected plants may produce fibrous roots above the lesions, but plants usually remain stunted. Symptoms of Rhizoctonia root rot may resemble symptoms caused by Fusarium root rot, and laboratory analysis to differentiate the two diseases may be necessary.
Persistence and Transmission
Generally, Rhizoctonia survives between crops as sclerotia or as fungal mycelia in the soil. Sclerotia are dense, compacted aggregates of dormant fungal hyphae, resistant to unfavorable environmental conditions. Sclerotia in infested soil or plant debris can be moved throughout the field by wind, rain, irrigation water, and farm implements. Once soil becomes infested, it will remain infested. The disease is most severe in cooler soils (around 60° to 65°F). Soil moisture conditions have little effect on disease severity. Young plants are more susceptible to infection than older plants.
Pythium Root Rot
Pythium root rot is caused by a group of soilborne fungi in the genus Pythium and results in seed rot and pre- and post-emergence seedling damping-off. Species of Pythium are found in all soils. Like other root rotting diseases, Pythium can cause poor plant stands, stunting, and discoloration of foliage.
Symptoms
Pythium attacks seeds and roots primarily. Infected seeds become soft and discolored. Diseased roots are characterized by colorless to dark brown, water-soaked lesions. Infected tissue is soft and watery and can be easily separated from the central cylinder of the stem by pulling the root between the thumb and index finger. Sometimes the stem is girdled. Occasionally, when beans are grown under irrigation or during cool wet periods, pods touching the soil are infected. They become water-soaked and covered with a fluffy white fungal growth. Symptoms of Pythium root rot may resemble Rhizoctonia root rot in some situations. In such cases, laboratory examination may be necessary to differentiate between the two diseases.
Persistence and Transmission
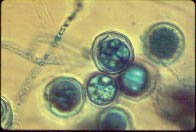
Figure 3. Protective, thick-walled oospore produced by Pythium
Many different species of Pythium can cause Pythium root rot. Each is adapted to slightly different environments, so it is difficult to identify temperature conditions which favor disease development. The disease can occur over a broad range of temperatures (60° to 90°F). Generally, Pythium root rot is most severe in wet soils. The fungus forms protective, thick-walled oospores that survive adverse environmental conditions in the soil or in crop debris (figure 3). Many species are capable of colonizing and surviving on dead plant material, so removal of host plants will not necessarily eliminate the problem. Oospores and infected plant material can be moved around the field by wind, rain, irrigation water, farm implements, and any other agent or process capable of moving soil. Once soil becomes infested, it will remain infested. Though Pythium root rot is most severe in young plants, the fungus can attack small roots, rootlets, and root hairs of older plants and reduce their productivity.
Fusarium Root Rot
Fusarium root rot on garden beans is caused by the fungus Fusarium solani f. sp. phaseoli. The fungus can attack older seedlings and is most severe on plants growing under stressful conditions.
Symptoms

Figure 4. Reddish- brown lesions and lengthwise cracks typical of Fusarium root rot on bean
Symptoms do not appear until a week or more after seedlings emerge. The first symptoms are narrow, long, red-to-brown streaks on the hypocotyls and taproot. The taproot later turns dark brown, and lengthwise cracks often develop (figure 4). The taproot may shrivel and die. Clusters of fibrous roots may develop above the shriveled taproot. These fibrous roots may keep the plant alive, and under ideal growing conditions, few aboveground symptoms will be observed. Infected plants may be stunted, have an unthrifty color, and grow more slowly than healthy plants as poor root function deprives them of nutrients and water. This can result in uneven plant stands.
Persistence and Transmission
The pathogen usually survives as thick-walled chlamydospores in soil. Spores germinate when stimulated by nutrients exuded by germinating seeds and root tips. Chlamydospores of Fusarium can germinate and reproduce near seeds and roots of many nonhost plants, as well as in organic matter. This means the pathogen can survive in the field indefinitely. The pathogen is moved around the field by wind, rain, irrigation water, farm implements, or by any other agent or process capable of moving soil. Plant damage is usually increased under environmental conditions that stress plants. These conditions include deep planting, soil compaction, hardpan layers, cool temperatures, high or low pH, low fertility, pesticide or fertilizer injury, and flooding or extended drought.
Controlling Root Rot Diseases
Controlling root rot diseases starts by providing conditions that promote plant health. Planting in a warm, well-prepared, well-drained, and well-fertilized seedbed capable of supporting rapid bean growth is the best method to prevent losses due to root-rotting diseases. Poor growing conditions, such as compacted soils or hard pan layers, need to be corrected in order to provide optimal growing conditions for your crop.
A crop rotation of at least 2 years with plants not in the legume family will help reduce losses to root rot. A rotation of at least 4 years with cereals or other nonhost crops is recommended for control of Rhizoctonia root rot.
Seed treatment or soil drench applications of fungicides containing the active ingredient metalaxyl can be used to control Pythium. Seed treatment or soil drench applications of fungicides containing the active ingredient pentachloronitrobenzene (PCNB) can be used to control Rhizoctonia root rot. Fungicides are generally not effective in controlling Fusarium root rot. Always follow the directions and restrictions on the manufacturer’s label. See Extension publications ANR-0500A or ANR- 0500B, Alabama Pest Management Handbook, Volume 1 (commercial vegetables) or Volume 2 (home gardens), for more information.
Soil solarization is a nonchemical method to sterilize soil and can be used to control root rots and other soilborne diseases of beans. See Extension publication ANR-0713, “Soil Solarization for the Control of Nematodes and Soil-Borne Diseases.” Contact your county agent for more information on this technique.
Root-Knot Nematodes
Root-knot (Meloidogyne spp.) nematodes (RKN) are the most common plant parasitic nematodes that damage fresh market beans in Alabama. Other nematode species such as root-lesion (Pratylenchus spp.), stubby root (Trichodorus spp.), reniform (Rotylenchus reniformis) and cyst (Heterodera spp.) are also found in fields and gardens but are not as damaging as RKN.
Symptoms
All plant parasitic nematodes that attack beans cause similar aboveground symptoms. Plants are often stunted. This rarely occurs uniformly over an extensive area because nematodes are usually distributed unevenly within a field or garden.
Leaves on infected plants are typically pale green to yellow in color and often wilt during the warmest part of the day (figure 5). Symptoms are most severe in light, sandy soils. High RKN populations can often result in reduced yields and occasionally the death of a plant.

Figure 5. Yellowing of lower leaves of snap bean caused by root-knot nematode damage. Galls on exposed roots are symptomatic of root-knot nematode
Belowground symptoms of RKN damage appear as galls, knots, or swellings (figure 5). Galls vary in size from the head of a pin up to 1⁄2 inch in diameter on larger roots. Galls cannot be removed because they are comprised of root tissue. Galls on severely infected roots may fuse, causing the root system to appear malformed. The disruption in normal root growth and root activity affects the plant’s ability to take up water and essential nutrients.
If a nematode problem is suspected but no galling is observed, another nematode species may be responsible. To confirm this, a soil sample from the affected area must be analyzed for nematodes by a diagnostic laboratory. See Extension publication ANR-0114, “Collecting Soil and Root Samples for Nematode Analysis.” Contact your county Extension agent for information on the proper way to take and ship a soil sample for nematode analysis.
Control
Planting a nematode resistant or tolerant variety is the most effective control method for RKN. The lima bean variety Nemagreen is considered resistant to RKN. The snap bean variety Harvester is considered tolerant to RKN. Other varieties that are resistant or tolerant to RKN may have become available after this report was published.
Rotations with nonhost crops is an inexpensive way of reducing damaging levels of nematodes. Because RKN has a wide host range, however, a grower is limited to what crops can be included in a rotation system. Usually, a grass crop such as corn is best. Clean fallowing is another option. Growers should also consider using a nematode-suppressive crop such as marigolds or velvetbean as a rotation alternative. See Extension publication ANR-0856, “Nematode Suppressive Crops.”
Other methods to control nematodes include soil solarization and the use of nematicides. See Extension publication ANR-0500A, Alabama Pest Management Handbook, Volume 1. Soil solarization can be used to sterilize the soil when environmental conditions are favorable for its use. See Extension publication ANR-0713, “Soil Solarization for the Control of Nematodes and Soilborne Diseases.” Contact your county Extension agent for more information on nematode suppressive crops and the proper use of a soil nematicide.
Aboveground Diseases
Rust of Garden Beans
Rust, caused by the fungus Uromyces phaseoli var. typica, attacks all aboveground plant parts, but is most commonly seen on the undersides of leaves. Rust is a common problem on snap beans but rare on lima beans in Alabama. Rust can cause significant yield losses when plants are attacked early in the season.
Symptoms

Figure 6. Rust colored pustules on bean leaf symptomatic of bean rust
Rust is most common on mature plants. It is usually observed on leaves but may also occur on pods. The initial symptoms of rust are small, white, slightly raised spots on the lower leaf surface. These pimple-like structures may be surrounded by a yellow halo. Within 1 week, these pustules break open into rust-colored lesions about the size of a pinhead (figure 6). The powdery mass of rust-colored spores will transfer the rust color to the fingers if rubbed across symptomatic leaves. These lesions will appear on the lower and upper leaf surface. About 1 week after the pustules appear, the entire leaf begins to turn yellow. Heavily infected leaves turn yellow, shrivel, dry up, and drop prematurely. A severely infected field looks scorched.
Persistence and Transmission
The rust fungus overwinters on dead, infected bean plants from the previous season. Spores are spread long distances by wind. Thousands of spores can be produced from each infected leaf, and a new generation of spores is produced about every 10 days. Disease development is favored by cloudy, humid weather and temperatures around 75°F.
Control
Control consists of crop rotation, sanitation, weed control, the use of resistant varieties, and the use of fungicides when necessary. Do not plant beans in the same field for at least 2 years. Eliminating crop debris after harvest and controlling volunteer bean plants during the rotation cycle will also aid in control.
Resistant varieties are available, but because of the large number of races of rust (more than 250 worldwide), selecting the best one for your area may be difficult. Some rust-resistant varieties include Raider, Rebel, Seminole, Provider, and Resisto.
Scout fields weekly and initiate a fungicide spray program when the first symptoms of rust appear. Use a fungicide with the active ingredient chlorothalonil on a 7-day spray schedule until the disease is no longer a problem. Always follow the directions and restrictions on the manufacturer’s label. See Extension publications ANR-0500A or ANR- 0500B, Alabama Pest Management Handbook, Volume 1 or 2, for more information.
Powdery Mildew
Powdery mildew, caused by the fungus Erysiphe polygoni, can be a serious disease of beans. It can affect all aerial plant parts.
Symptoms
Found primarily on older leaves of plants, this disease forms small, round, whitish spots on the older leaves. On careful examination, a cottony fungal growth can be seen in the white spots. The leaf surface underneath these growths is dark and mottled. Eventually, the entire leaf will be covered with a talc-like powder. In severe cases, the leaf will become distorted and yellow and may die prematurely, resulting in extensive defoliation. Stems and pods can also be infected. Infected pods develop purplish spots and may become distorted.
Persistence and Transmission
It is not known how this fungus overwinters. Some workers believe it survives on weeds year-round. A large part of the talc-like powder seen on infected leaves is composed of fungal spores which are easily dispersed by wind or splashing rain.
Unlike other fungal diseases, powdery mildew can become severe under conditions of low rainfall and low humidity. The disease occurs primarily on older leaves and usually appears later in the growing season when there is little threat to productivity.
Control
Control consists of the use of resistant varieties, use of fungicides when necessary, and good plant nutrition. It is important to maintain a balanced soil fertility program because plants grown under nutritional stress are more susceptible to powdery mildew.
The snapbean variety Contender has resistance to powdery mildew. Powdery mildew only needs to be controlled during flowering and early pod set. The disease has little effect on yield when it occurs after this time.
Use fungicides containing sulfur to control powdery mildew. Always follow the directions and restrictions on the manufacturer’s label. See Extension publications ANR-0500A or ANR-0500B, Alabama Pest Management Handbook, Volume 1 or 2, for more information.
Downy Mildew
Downy mildew is caused by the fungus Phytophthora phaseoli. The disease can cause heavy losses in lima beans when weather conditions favor its development.
Symptoms
Downy mildew is easily recognized by the white, cottony fungal growth that forms on pods. A reddish-brown or purple border may develop around the infected area. Infected pods shrivel, die, and turn black, and often remain attached to the plant. The fungus may penetrate through the pod and infect the seeds.
Young shoots, flowers, and leaves are also affected. Leaf veins may become purple and distorted. The disease can cause seedling damping-off.
Persistence and Transmission
The downy mildew fungus survives the winter in infected seed and in plant debris (vines and pods) left in the field. Both seed and dead plant material can act as sources of disease the following year.
Disease development is favored by wet weather with cool nights, heavy dews, and fairly warm days. Infection is most severe on pods lying close to the soil. Susceptible plants can be destroyed by downy mildew in a few days under favorable conditions. The disease is most commonly spread by wind and rain.
Control
The most effective control for downy mildew is the use of resistant varieties. The lima bean variety Eastland is resistant to the disease.
Growers should avoid using seed from a crop previously infected with downy mildew and should not plant beans in the same field for at least 2 years.
Scout fields weekly for disease. Initiate a spray program with a fungicide containing the active ingredient chlorothalonil when the disease is first observed, and continue on a 7- to 10-day schedule. Always follow the directions and restrictions on the manufacturer’s label. See Extension publications ANR-0500A and ANR-0500B, Alabama Pest Management Handbook, Volume 1 and 2, for more information.
Anthracnose on Garden Beans
Bean anthracnose, caused by the fungus Colletotrichum lindemuthianum, occurs commonly when locally grown seed is used in home gardens. The disease can cause 100 percent yield reductions when prolonged cool, wet weather conditions persist during the growing season.
Symptoms
Anthracnose symptoms may show up on leaves, stems, pods, and seeds. Lesions on stems are sunken, oval cankers extending up and down the stem. Cankers range in color from brown to black with purple to brick-red borders, giving the lesion an eyespot appearance. Bean stems weakened by cankers are often more susceptible to damage from cultivation and strong winds.
Lesions are more common on leaf petioles and on lower surfaces of leaves and leaf veins. Lesions are elongated, angular, and brick-red to purple, becoming dark brown to black with time (figure 7). Lesions often follow veins on the lower leaf surface. When severe, angular dead zones may form on the upper leaf surface giving the plants a ragged appearance.
The most easily recognized symptom of anthracnose on beans occurs on the pods. Small, reddish-brown elongated spots form initially. These spots gradually become somewhat circular, sunken at the center, and vary in color from tan to brown to reddish-brown to black (figures 8 and 9). A rusty-brown, slightly raised border forms around each lesion. Older lesions may be over 1⁄4inch in diameter. During wet weather, a mass of pinkish-colored spores can be seen on the lesions. Young pods may shrivel and dry if severely infected. The fungus can invade the pod and infect developing seeds. Seed infected with the disease have dark, sunken lesions of various sizes that may extend through the seed coat. When infected seeds are planted, the fungus will multiply, form lesions on the cotyledons, and eventually spread to other plant parts during the season.
- Figure 7. Reddish-purple leaf lesions caused by bean anthracnose
- Figure 9. Small, reddish-brown elongated spots are symptomatic of anthracnose on bean pods
Persistence and Transmission
Anthracnose overwinters inside bean seed and in infected bean debris left in the field after harvest. The fungus can survive in seed as long as the seed remains viable, and for more than 2 years in old bean debris in the field. The fungus is spread by wind, rain, animals, workers, and farm implements. Cool, wet weather promotes disease development while the fungus ceases to be active during hot, dry conditions.
Control
The most effective methods to control bean anthracnose include using disease-free seed, rotating crops, and following a fungicide spray program initiated through a disease scouting program.
Seed produced under wet, humid conditions are most susceptible to infection. For this reason, it is best not to save seed from year to year in Alabama. Do not grow beans in the same field more often than once every 3 years. Do not work fields when plants are wet because fungal spores are easily spread from diseased to healthy plants under these conditions.
Scout fields weekly for symptoms of the disease. When the disease is first observed, apply a fungicide with the active ingredient chlorothalonil. Reapply weekly during the season. Always follow the directions and restrictions on the manufacturer’s label. See Extension publications ANR-0500A or ANR-0500B, Alabama Pest Management Handbook, Volume 1 or 2, for more information.
Viral Diseases
Viral diseases are common in snap and lima beans. The viruses affecting beans in Alabama fit into a group known as the mosaic viruses. The two most common viruses affecting garden beans are bean common mosaic virus (BCMV) and bean yellow mosaic virus (BYMV). At least ten strains of BCMV have been identified. Other viral diseases that can occur in Alabama include cucumber mosaic virus, southern bean mosaic, and peanut mottle virus.
Symptoms

Figure 10. Plant infected with a mosaic virus exhibiting leaf mottling
Symptoms caused by BCMV and BYMV are similar and difficult to distinguish in the field. Symptoms can vary with variety, age of plants, virus strain, and environmental conditions. Both viruses cause stunting and yield reduction. Leaves on infected plants exhibit a mosaic or mottled discoloration resulting in irregular light green and yellow patches intermingled with healthy dark green tissue (figure 10).
Bean plants infected with BCMV early in the season are typically stunted and have mottled leaves. Leaves may be longer and narrower than healthy leaves and may show some puckering or downward curling at the margins.
Plants infected with BYMV are more stunted and bushy. Pods on infected plants are undersized and sometimes curled. Overall bean production is reduced. The contrast between the yellow and green areas on mottled leaves is more intense with BYMV than with BCMV.
Plants infected by either virus early in the season often produce a small crop. Symptoms are less severe on plants infected later in their development, and a near normal crop is often produced.
Persistence and Transmission
BCMV and BYMV are commonly spread by aphids. Aphids pick up the virus on their mouth parts when they feed on infected plants. They spread the virus when they move to healthy plants and continue to feed.
Both viruses can also be spread mechanically by leaves rubbing against one another or by workers handling healthy plants after working with infected plants.
BCMV can also be transmitted through seed, which allows for long-distance spread between production areas and survival from season to season. BYMV has a much wider host range then BCMV but is not seed transmitted. BYMV can infect most species of the legume family and may overwinter in one of these alternate hosts.
Control
The use of resistant bean varieties is the most effective and least expensive control practice available to manage BCMV. A number of snap bean varieties have BCMV resistance. These include Bush Blue Lake, Contender, Empress, Harvester, Lake Superior, Larma, Milkado, Provider, Rebel, and Triumph. There are no snap bean varieties available at this time with resistance to BYMV. There are no lima bean varieties available with resistance to BCMV or BYMV. Using virus-free seed and controlling aphids may reduce disease spread.
Bacterial Diseases
Two bacterial diseases that commonly occur on snap and lima beans in Alabama are common bacterial blight, caused by Xanthomonas phaseoli, and halo blight, caused by Pseudomonas syringae pv. phaseolicola. Though caused by different species of bacteria, each produces similar symptoms on bean plants.
Symptoms
The initial symptom of bacterial blight infection appears as water-soaked spots on the lower leaf surface. Lesions usually turn brown with time and often are surrounded by a narrow yellow halo. As the lesions enlarge, they often run together resulting in extensive dead areas. When the infection is severe, leaves often appear burned. In time, leaves become ragged and may drop prematurely.
Symptoms on pods appear as circular, sunken, reddish-brown lesions that vary in size. Pod lesions are usually surrounded by a dark, reddish-brown margin. Infected seed are usually shriveled and discolored, and exhibit poor germination and vigor.
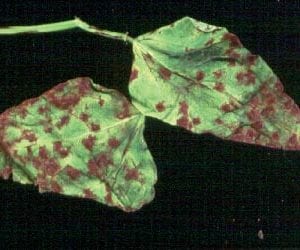
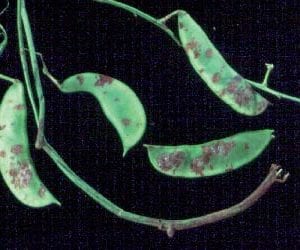
The initial symptom of halo blight also appears as water-soaked spots on the lower leaf surface. A distinctive greenish-yellow halo forms around the initial infection site which remains relatively small (figure 11). When severe, the leaves take on a yellow appearance that may be misidentified as a nutritional or pH disorder. Pod symptoms generally consist of a reddish-brown discoloration that may also appear water-soaked (figure 12). As pods mature and turn yellow, pod lesions remain green and may exhibit a crusty bacterial ooze on their surfaces. Infected seed are often shriveled and discolored.
- Figure 11. Yellow halo surrounding infection site on bean leaf typical of halo blight
- Figure 12. Water-soaked spots on bean pod infected with halo blight
Persistence and Transmission
Spores of bacterial blight and halo blight can survive inside bean seed and in plant debris in the soil. Seed contamination is an effective means of local and widespread dissemination of the pathogens. Seedlings arising from infected seed harbor large numbers of bacteria. Both pathogens can survive on alternate weed hosts and volunteer bean plants in an area.
The bacteria can survive for about a year in plant debris. Survival is generally longer in plant residue left at or near the soil surface. High humidity, rain, or both favor rapid development of the disease in the field. Bacterial spores are spread by windblown rain, splashing irrigation water, or contact between adjacent leaves wet from rain or dew.
Development of bacterial blight is favored by temperatures in the 85° to 95°F range. Halo blight is considered a cool-season disease favored by temperatures in the 65° to 75°F range.
Control
Start control measures by using certified disease-free seed. Do not save seed from the previous season if bacterial blight or halo blight was a problem.
Use crop rotation. Do not plant any bean crop in the same field for at least 2 years. Control volunteer beans, and eliminate weeds that may act as reservoir hosts for the bacteria.
Finally, if the disease is present and weather conditions favor its development, a copper-type fungicide/bactericide may be needed. Always follow the directions and restrictions on the manufacturer’s label.