Fish & Water


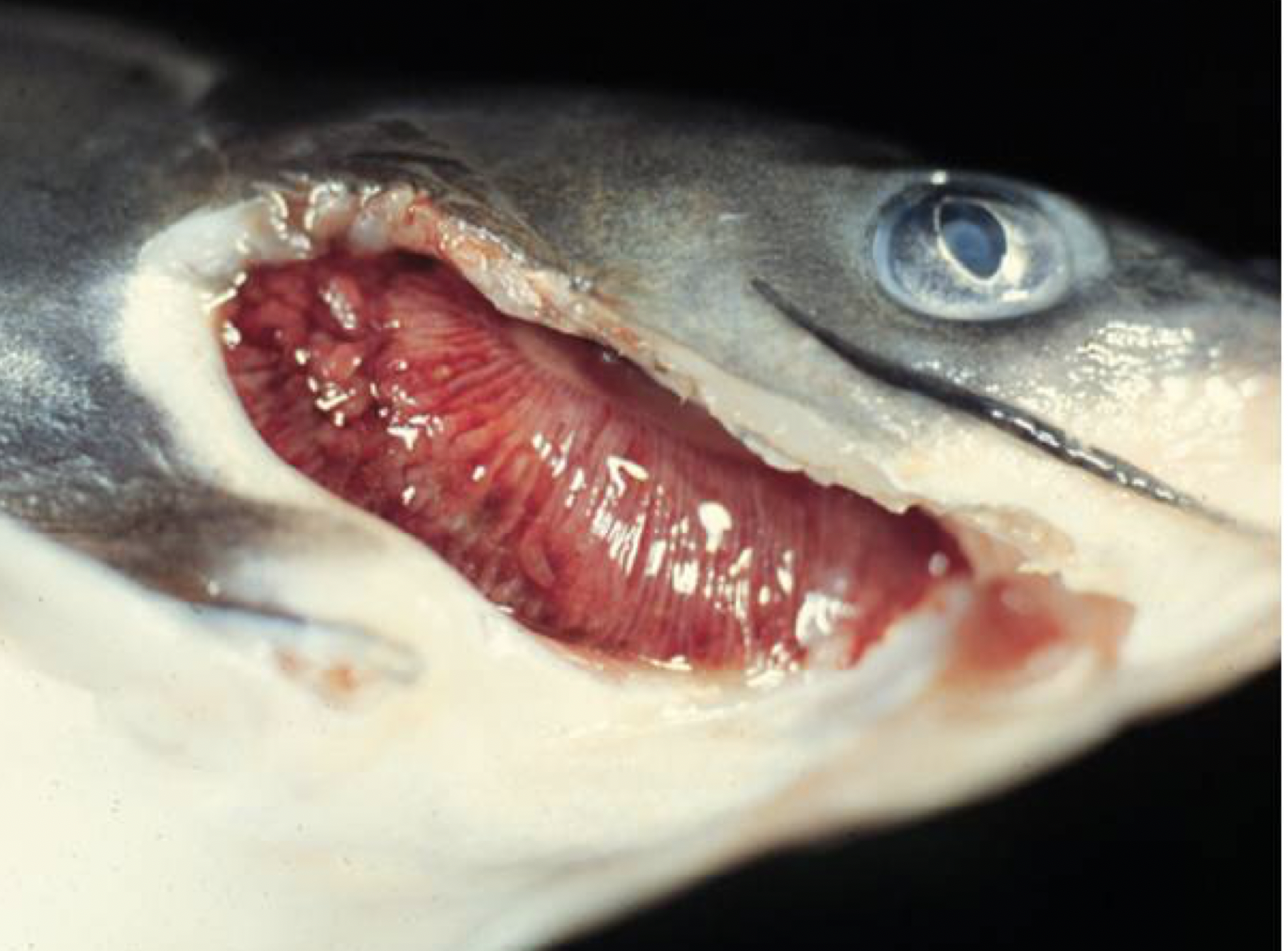
Figure 1. The swollen gills of a channel catfish that has been diagnosed with proliferative gill disease (PGD). Note the hamburger like appearance. (Photo courtesy of John Hawke, Louisiana State University)
Proliferative gill disease (PGD), also known as hamburger gill disease (figure 1), is the most significant parasite disease of farm-raised channel catfish and to some extent hybrid catfish.
Blue catfish, Ictalurus furcatus, are essentially immune to PGD and blue × channel catfish hybrids are partially immune and may prevent the PGD parasite from completing its life cycle. Therefore, channel catfish (and sometimes their hybrids with blue catfish) are the only species susceptible to PGD.
Caused by Parasite
Proliferative gill disease is caused by a myxosporean parasite that results in severely swollen gills with broken gill cartilage. Most cases of PGD will occur between March and May when the ideal water temperatures for this disease occur (61 to 77 degrees F). Mortalities are often significant and can exceed 50 percent. The high mortality rate is due to the severe gill swelling, making it difficult for the fish to extract enough dissolved oxygen from the water. This essentially suffocates the fish. The swelling and red hemorrhagic areas next to white dead gill tissue give the gills a ground hamburger meat look (featured image above).
The parasite causing PGD is Henneguya ictaluri, a spore-forming parasite that alternates between two hosts, an annelid worm and channel catfish. The annelid worm, Dero digitata, which lives in pond mud, hosts the actinospore, Aurantiactinomyxon ictaluri. The actinospore develops in the worm, which then releases the actinospore stage into the water through its feces. This actinospore floats in the water with its three winglike projections that act as buoyancy floats. The actinospore attaches to the fish’s gills and releases its plasmodial sporoplasm, which contains infectious cells, into the fish gills causing a severe inflammatory response leading to respiratory distress.
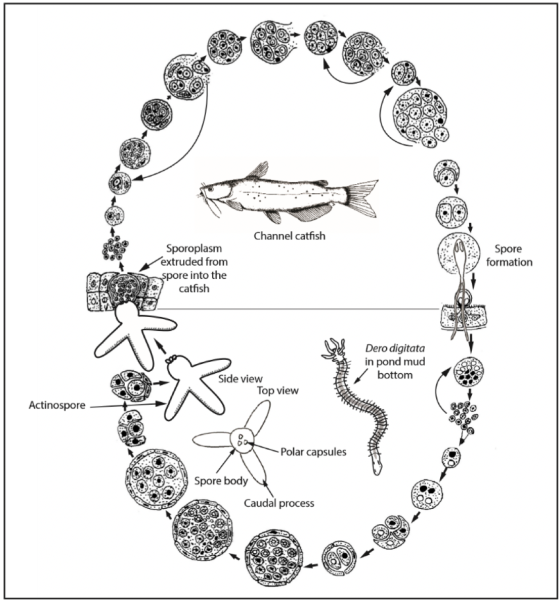
Figure 2. Life cycle of Henneguya ictaluri, causative organism of PGD, showing the development of its myxospore stage in channel catfish and actinospore stage in Dero digitata. (Photo courtesy of Wyvette Williams, Kentucky State University)
This inflammatory response leads to swelling or a ground hamburger appearance of the gill. It occurs most often in younger fish, especially those stocked into new ponds, but older fish and established ponds are affected at times. Multicellular spores form within the plasmodia inside the fish gills. Some mature into Henneguya ictaluri spores, which are released into the pond environment and are ingested by the Dero worms. This completes the life cycle (figure 2).
Management Options
There is no treatment for PGD, but some methods have demonstrated anecdotal success. Since PGD is considered a new-pond disease, new or renovated ponds should be filled partially with water from adjacent, established ponds. Since the Dero worm is a host, stocking smallmouth buffalo, Ictiobus bubalus, that consume the worms could reduce the number of hosts for PGD.
The prevention of PGD has been accomplished by applying hydrated lime to dry ponds before filling. The caustic nature of hydrated lime and the associated pH change in the pond soil significantly reduce the amount of Dero worms present in the mud before filling.
As for treatment, catfish farmers have often pumped water from an established, healthy pond into a pond infected with PGD with some degree of success in reducing mortalities. Note that when PGD-infected catfish are removed from the infected pond, they recover quickly. Although no proven treatments exist, once a PGD outbreak is over, there does not tend to be a recurrence of the disease in that pond for the rest of that season.