Fish & Water

Waterborne illness because of drinking untreated water can be a product of both municipal and private water systems. While awareness of drinking water quality has increased considerably, awareness regarding private well health still lags. Private well owners benefit tremendously from regularly testing their well water. An alternate drinking source or treatment plan should be established if the well water contains contaminant concentrations greater than the maximum contaminant levels set by the EPA.
Well water should be screened for a variety of contaminants, including nitrate, iron, fecal coliform bacteria, total dissolved solids, and sulfate. It is also important to assess pH, alkalinity, and hardness. If there is a specific contaminant of concern in the area (like arsenic or volatile organic compounds), well owners should seek appropriate testing methods. These are just some of the tests recommended for well water quality, and it is at the discretion of the owner of the well to seek extensive testing regarding their drinking water quality.
Private well health encompasses more than just quality assurance. A responsible well owner should review the well construction standards, location recommendations, and maintenance schedule to get a thorough understanding of their well.
Common Contaminants
Basic Water Measurements
Total dissolved solids. Total dissolved solids (TDS) are a measure of dissolved minerals in the water, including salts, metals, cations, and anions. TDS has a secondary contaminant limit (SMCL) because it can stain home fixtures and cause water to taste salty. The concentration of TDS is often dependent on the geographic location but can be elevated by sewage, urban or agricultural runoff, saltwater intrusion, or water treatment chemicals.
Hardness. Hardness is generally the amount of dissolved calcium and magnesium in the water, but it can include other particles such as iron or manganese. Hardness does not have a mandated MCL, but it can cause a scale to form that clogs pipes and can make it difficult for soap to lather. Surrounding geology largely affects water hardness, but substances like gypsum can increase hardness.
pH. Water pH describes the acidity or alkalinity of the water. pH does not have a limit, but natural waters tend to be between 6 and 9. Elevated acidity can cause leaky pipes, leach metals from plumbing fixtures, or cause a bitter taste. Elevated basicity can corrode fixtures and cause a bad taste.
Organic materials. Organic materials are substances left from once-living organisms. If wells are left open, materials like leaves can fall in and affect taste and color. When organic materials decompose, they can form a gas called hydrogen sulfide. This gas smells like rotten eggs and may stain or corrode pipes and fixtures.
Metals
Metals are naturally occurring in the environment and can dissociate into groundwater. The severity of their effects on drinking water quality varies based on the metal present and its concentration. Heavy metals are a group of metals that have a high atomic weight and have been distributed in the environment from industrial, agricultural, medical, domestic, and technological applications. Their toxicity depends on the dose, route of exposure, and demographics of the exposed individual. Heavy metals may include lead, arsenic, and uranium.
Lead. Lead is a primary contaminant and can cause physical and mental development delays, kidney problems, or high blood pressure. In older systems where lead pipes are used, lead can enter the drinking water if the pipes corrode.
Copper. Copper has both primary and secondary contaminant levels. Above the secondary limit, copper can leave blue-green stains or impart a metallic taste. Above the primary limit, copper can cause gastrointestinal distress, kidney damage, or liver damage. Copper can enter your drinking water from natural geologic deposits or through pipe corrosion.
Aluminum. Aluminum is a secondary contaminant that can impart an undesired color. Aluminum may be found in waters with low pH.
Iron and manganese. Iron and manganese are secondary contaminants that can cause undesired tastes, colors, and odors. Iron tends to impart a red color or stain, while manganese leaves a brownish-black color. High concentrations of these elements can create a suitable habitat for iron and manganese bacteria, which can produce slime and clog pipes.
Silver and zinc. Silver and zinc are secondary contaminants, and above the secondary MCL, silver can discolor organs, skin, and hair, while zinc can impart a metallic taste and cause damage to the pancreas.
Pathogens
Coliform bacteria. Coliform bacteria are often used as an indicator for the presence of pathogens in water. Coliforms can be found naturally in plants and soil, and many are not harmful. However, coliforms are also found in the digestive tract of warm-blooded animals. If a water source tests positive for coliforms, another test should follow to check specifically for fecal coliforms. A positive fecal coliform or generic E. coli test suggests the supply is contaminated with fecal matter and is unfit for consumption.
E. coli. One of the most common waterborne bacterial pathogens people will hear about is E. coli. Not all E. coli is harmful, but strain 0157:H7 can cause gastrointestinal distress and vomiting. A common issue in surface waters, E. coli can be a result of leaky sewer lines or sewer runoff, a malfunctioning septic system, or an unprotected wellhead. Other waterborne bacterial pathogens include Salmonella and Vibrio cholerae.
Viruses. The small size and survival abilities of viruses allow them to travel in aquifers and can make them a serious threat to groundwater quality. Waterborne viral pathogens include adenovirus, norovirus, and rotavirus.
Other Contaminants
Nitrate. Nitrate is a primary contaminant that is seen in agricultural production but is undesirable in drinking water. Nitrate can enter water sources through fertilizer leaching, septic system discharge, municipal waste, and animal waste. Nitrate has a maximum contaminant level, as it can reduce the ability of hemoglobin to transport oxygen, causing shortness of breath. This condition is known as blue-baby syndrome and can be fatal to infants.
Fluoride. Fluoride is a primary contaminant that is beneficial at low levels and is often added to drinking water supplies to prevent tooth decay. However, the concentration can be elevated by geologic deposits or manufacturing discharge. When the concentration exceeds the MCL, fluoride can cause bone disease and mottled teeth.
Arsenic. Arsenic is a primary contaminant known for its high toxicity. Arsenic can be found in some pesticides, fungicides, and wood preservatives. Arsenic is also a product of mining and coal processing activities, as it is naturally found in rocks and soils. Consuming arsenic over time can cause increased cancer risk, enlargement of the liver or spleen, and the development of skin lesions or growths.
Radon. Radon is a radioactive, colorless, odorless, and tasteless gas that can dissolve in water and accumulate in confined spaces like wells. Uranium is found naturally in soils and rocks, and radon is produced when uranium is broken down. When contaminated water is collected from a tap, some radon will escape into the air. Breathing in radon can cause lung cancer and consuming can cause cancer of internal organs.
Pesticides. Pesticides are not often found at elevated concentrations in drinking water sources, as modern pesticides are regulated and usually degrade naturally. However, wells sourced from shallow aquifers in areas with high soil infiltration could be at risk, especially if sealed improperly. Health effects depend on the pesticide but can include cancer risk, skin or eye irritation, or nervous system damage.
Anthropogenic Sources of Contamination
Anthropogenic contamination is contamination caused by humans. Many industrial, commercial, residential, and agricultural activities require care to avoid contamination of groundwater. The Alabama Department of Environmental Management (ADEM) has listed underground storage tanks and failing septic systems as considerable threats to Alabama’s groundwater because of their widespread presence.
Underground storage tanks are used to safely store substances like gasoline or oil, however, if the tank malfunctions contaminants can leak out and leach into groundwater sources.
Septic systems are used to treat residential wastes and protect water quality when sewer systems are not present. With proper installation and use, this is accomplished by bacterial breakdown of wastes and filtration through soil. Once in use, behavior such as flushing oil or cleaners down the toilet can harm your septic system’s bacteria and lead to contamination of water sources with human waste.
Pesticides are not often found at elevated concentrations in drinking water sources. Modern pesticides are regulated and usually degrade naturally. However, wells sourced from shallow aquifers in areas with high soil infiltration could be at risk, especially if sealed improperly.
Unregulated dumping activities create a risk for shallow aquifers on dead-end roads, streams, and sinkholes that are sometimes turned into makeshift dumping sites for garbage, dead animals, and hazardous materials. This is an eyesore that can attract disease carrying insects and rodents, while also contaminating surface waters and groundwater. Unwanted material should be properly disposed of in landfills containing protective liners required by the Resource Conservation and Recovery Act.
Natural Susceptibility
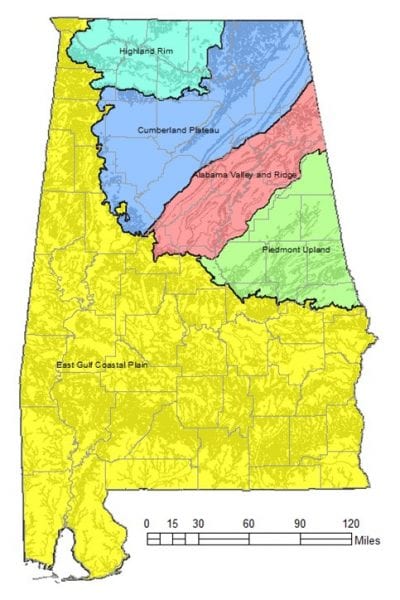
Geological Survey of Alabama
Image credit: https://www.gsa.state.al.us/
Alabama is broken into five physiographic regions that may be susceptible to different forms of contamination due to their different locations and geology.
The Highland Rim Region
The Highland Rim region in North Alabama contains aquifers with high amounts of limestone, resulting in the formation of caves and sinkholes. These open areas make it easy for contamination to travel from the ground surface into aquifers. The groundwater in this region is typically hard and can contain high iron, carbon dioxide, and hydrogen sulfide concentrations.
The East Gulf Coastal Plain Region
This region has several wells located near saltwater, which increases the risk for saltwater intrusion. This occurs when coastal aquifers are heavily pumped, causing saltwater to be drawn in.
The Valley and Ridge Region
The valleys in this region formed when areas comprised of easily erodible rocks were worn down, and areas comprised of strong, erosion-resistant rocks remained. The highly erodible rocks of the valley allow fast infiltration, making the aquifers susceptible to surface pollution.
The Cumberland Plateau Region
The Cumberland Plateau region has multiple groundwater sources, including the Pottsville Formation, the Bangor Limestone, and Hartselle Sandstone. Water from the Pottsville Formation can exceed the SMCLs for iron, resulting in stains or bad taste. Water from the Bangor Limestone and Hartselle Sandstone is generally hard.
More Information
Need your well water tested? See Where to Get Your Well Water Tested for more information about testing well water in Alabama. More information for private well owners is available on the Private Well Program page on the Alabama Extension website, www.aces.edu.

