Crop Production

Phosphorus (P) is essential to all forms of life on this planet. It is an essential nutrient necessary for growth and development of plants and animals on which our food supply depends.
Phosphorus constitutes about 0.2 percent of a plant’s dry weight, where it is primarily a component of tissue molecules such as nucleic acids, phospholipids, and adenosine triphosphate (ATP). After nitrogen (N), phosphorus (P) is the second most limiting nutrient. It can reduce plant growth and development and potentially limit crop yield. However, excess phosphorus in soil can be detrimental to the environment because it can enter freshwater bodies through surface runoff and can cause algal bloom reducing water quality. Improved phosphorus management can create profitable crop production systems while reducing negative impacts on the environment. The objective of this document is to understand phosphorus forms, transformation, and cycling in the soil. Phosphorus cycle is unique and different from the nitrogen cycle because phosphorus does not exist in a gaseous form. This document provides basic information on the various forms of phosphorus present in the soil and the processes that affect phosphorus availability for crop production.
Phosphorus Forms Present in the Soil
Soil phosphorus is found in two forms, namely organic and inorganic (figure 1). These two forms together make up the total soil phosphorus. Although total soil phosphorus is generally high, with concentrations ranging from 200 to 6,000 pounds per acre, 80 percent of this phosphorus is immobile and not available for uptake by the plant.
Approximately 30 to 65 percent of total soil phosphorus is in organic forms, which are not plant available, while the remaining 35 to 70 percent is in inorganic forms. Organic forms of phosphorus include dead plant/animal residues and soil micro-organisms. Soil micro-organisms play a key role in processing and transforming these organic forms of phosphorus into plant available forms. The inorganic phosphorus forms can be classified to exist in three different pools:
- Plant-available (soil solution) phosphorus: This pool is comprised of inorganic phosphorus dissolved in water/soil solution that is readily available for plant uptake.
- Sorbed phosphorus: This phosphorus pool is comprised of inorganic phosphorus attached to clay surfaces, iron (Fe), aluminum (Al), and calcium (Ca) oxides in soil. The phosphorus in this pool is released slowly for plant uptake.
- Mineral phosphorus: This phosphorus pool is comprised of primary and secondary phosphate minerals present in soil. Examples of primary phosphorus minerals include apatite, strengite, and variscite. The secondary phosphorus minerals include calcium (Ca), iron (Fe), and aluminum (Al) phosphates. The release of phosphorus from this pool is extremely slow and occurs when the mineral weathers and dissolves in soil water.
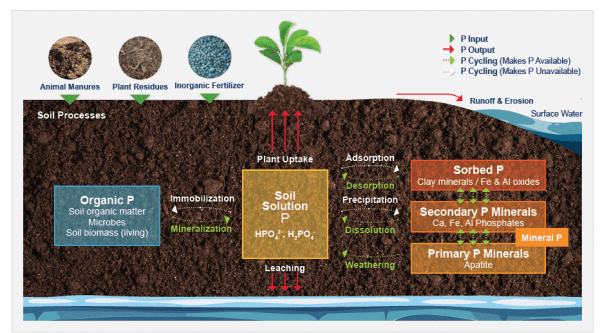
Figure 1. Soil phosphorus cycle. This figure illustrates the sources of phosphorous inputs in the soil, pathways through which phosphorus becomes available/ unavailable for plant uptake, and phosphorus outputs/ loss pathways.
Phosphorus Cycling and Transformation in the Soil
Once phosphorus enters the soil through chemical fertilizers (inorganic source), manure, biosolids, or dead plant or animal debris (organic sources), it cycles between several soil pools via processes such as mineralization, immobilization, adsorption, precipitation, desorption, weathering, and dissolution. Following are explanations of these processes:
Mineralization and Immobilization
Mineralization is a process through which organic phosphorus in soil is converted into inorganic phosphorus with the help of soil microbes. Immobilization, on the other hand, is the reverse of mineralization. During immobilization, inorganic phosphorus forms are converted back to organic forms and are absorbed into the living cells of soil microbes. Immobilization typically occurs when crop residues are incorporated in the soil. As crop residues decompose, more phosphorus becomes available in the soil solution through mineralization. Because mineralization and immobilization processes are biological processes, they are highly influenced by soil moisture, temperature, pH, organic carbon to organic phosphorus ratio of crop residues, microbial population, etc.
Adsorption and Desorption
Adsorption is a process in which phosphorus present in soil solution is attached/bound to the surface of soil particles. The phosphorus binding takes place on clay surfaces or the iron (Fe) and aluminum (Al) oxides and hydroxides present in soil. Adsorption is a fast process and reversible in nature, meaning that adsorbed phosphorus can be released into soil solution via a process known as desorption and will be available for plant uptake.
Soils containing greater concentrations of iron and aluminum oxides have greater potential to adsorb phosphorus than soils with relatively low iron and aluminum oxides. Another soil property that favors phosphorus adsorption is the clay content. Soils with greater clay content have higher adsorption capacity than coarse textured sandy soils.
Weathering, Precipitation, and Dissolution
Soil contains minerals that are rich in phosphorus. These minerals are classified into primary and secondary minerals. Minerals break down over time (a process referred to as weathering) and release phosphorus in the soil solution for plant uptake. Primary minerals such as apatite are very stable and resistant to weathering. Hence, phosphorus is released very slowly compared to secondary phosphorus minerals such as calcium, iron, or aluminum phosphates.
Precipitation on the other hand is a process by which metal ions such as Al3+ and Fe3+ (these ions are dominant in acidic soils) and Ca2+ (dominant in calcareous soils) react with phosphate ions present in the soil solution to form minerals such as Al-, Fe-, or Ca-phosphates. Precipitation is a slow process and involves a permanent change into metal phosphates. These metal phosphates can release phosphorus in soil solution upon dissolution, but the release rate is very slow.
Dissolution is a form of weathering when the phosphate minerals dissolve and release phosphate back into the soil solution.
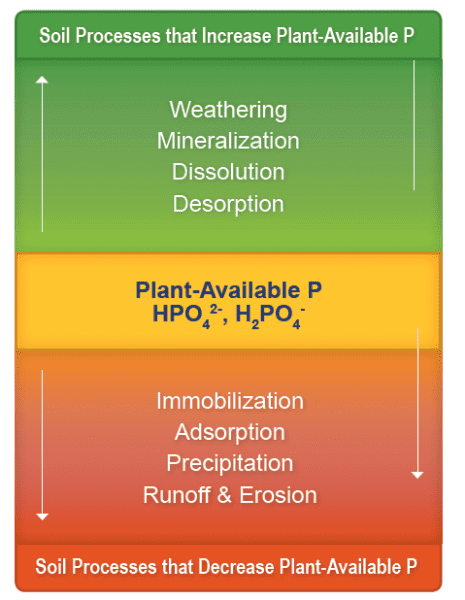
Figure 2. Soil processes that affect phosphorus availability for plant uptake.
Phosphorus Loss
Phosphorus is removed from soil by (a) crop/plant uptake, (b) runoff and erosion, and (c) leaching (figure 1). Surface runoff is the major pathway for phosphorus loss from soils. Runoff water carries away both soluble (dissolved) phosphorus and particulate (eroded soil particles) phosphorus from soil surface. Leaching is the loss of soluble phosphorus from sub-surface soil as water percolates vertically down the soil profile. In general, phosphorus loss by leaching is minimal compared to surface runoff.
Factors Influencing Phosphorus Availability in the Soil
While the processes such as weathering, dissolution, mineralization, and desorption increase phosphorus availability in the soil for plant uptake, processes such as immobilization, adsorption, precipitation, runoff, and erosion decrease the phosphorus availability (figure 2).
In addition, phosphorus availability in soil solution is influenced by the following factors:
- Organic Matter. Organic matter is an important factor in controlling phosphorus availability. With the addition of organic matter, availability of phosphorus increases.
- This is due to the following reasons:
- Mineralization of organic matter releases plant- available forms of phosphorus into soils.
- Organic molecules will compete with phosphate adsorbed to soil surfaces and will reduce phosphorus retention. This process will increase availability of phosphorus.
- This is due to the following reasons:
- Clay Content. Soils with higher clay content have high phosphorus retention capacity because clay particles have very large surface area per unit volume, which can adsorb phosphorus easily.
- Soil Mineralogy. The mineral composition of the soil influences the phosphorus adsorption capacity. For example, soils with a high content of Al3+ and Fe3+also tend to have the greatest phosphorus adsorption capacity.
- Soil pH. Optimum soil pH between 6 and 7 will result in maximum phosphorus availability. At low pH (acidic soils), soils have greater amounts of aluminum and iron, which form very strong bonds with phosphate. At high pH when calcium is the dominant cation, phosphate tends to precipitate with calcium.
- Other factors. Temperature, moisture, and soil aeration can affect the rate of P mineralization from organic matter decomposition. For example, in warm, humid climates organic matter decomposes faster compared to cool dry climates.
Summary: Phosphorus in a Nutshell
Various components of phosphorus cycle in soil can be correlated with the types of money in your bank. Just as money can be separated into categories—savings or checking accounts, the checks you carry for use as needed, and the cash you keep with you—phosphorus in soil can also be categorized to exist in three different accounts/pools (figure 3).
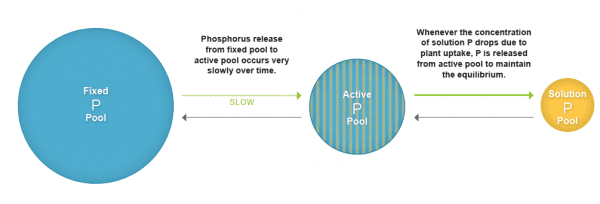
Figure 3. Phosphorus pools in the soil.
The first pool (savings /checking account) is called fixed or nonlabile pool. This phosphorus pool is largest of all the pools. This phosphorus is unavailable for plant uptake and is comprised of primary minerals (insoluble inorganic phosphate compounds) and organic phosphorus compounds that do not mineralize easily.
The second pool (checks you carry) is known as the active or labile phosphorus pool. This pool consists of adsorbed phosphorus, secondary phosphate minerals, and organic phosphorus that mineralizes easily.
The third pool (cash that you carry with you) is the smallest of the pools and comprised of inorganic phosphates and a small amount of organic phosphorus. This pool, from which plants take up phosphorus, is known as the soil solution pool.
These three pools exist in equilibrium with each other. As plants remove phosphorus from soil solution, phosphorus is replenished by the active pool. Similarly, as phosphorus concentration in active pool decreases, phosphorus is released from fixed pool to the active pool very slowly over time. The concentration of phosphorus available to plants at any time is very low and ranges from 0.001 mg L-1 to 1 mg L-1. The forms of phosphorus most readily accessed by plants are orthophosphate ions (H2PO4–, HPO42-) whose availability depends on soil pH. Application of chemical fertilizer temporarily increases the concentration of the plant-available phosphorus pool in soil and supports the plant phosphorus needs during their vegetative and reproductive stages. It is always a good practice to check the status of phosphorus in soil through regular soil testing before applying phosphorus fertilizers. Addition of phosphorus beyond the agronomic need of crops has minimal effect on crop yield. However, the excess phosphorus is susceptible to loss through runoff and erosion and can promote algal growth in freshwater systems causing the degradation of water quality.

